Abstract
Chronic myeloid leukemia (CML) has become a chronic disease, for which the chronic phase is manageable with tyrosine kinase inhibitor (TKI) therapy. Patients with optimal responses to TKIs have achieved long‐term survival, and treatment‐free remission (TFR) has since become an additional treatment goal in CML. In this review, we discuss important factors to consider prior to stopping treatment. In addition, published and presented data with the first‐generation TKI imatinib, as well as current clinical trials evaluating TFR with the second‐generation TKIs dasatinib and nilotinib, are examined. Results obtained outside of clinical trials have been included as well. Because successful TKI discontinuation depends upon accurate BCR‐ABL1 monitoring, emerging technologies are also discussed. Clinical data obtained to date indicate that for many patients who achieve deep molecular response (DMR) on TKI therapy, TFR is a safe treatment goal, and, if the response is lost, patients can expect to regain their responses immediately upon reinitiation of TKI. It is also clear that there remains much room for improvement to make TFR a successful reality for most patients. Data from ongoing trials should help refine decisions as to which patients are the best candidates to attempt TKI discontinuation with safe monitoring in place.
1. INTRODUCTION
Chronic myeloid leukemia (CML) has become a chronic disease—initially, for some patients, with the use of interferon alpha,1 but primarily with the routine use of tyrosine kinase inhibitor (TKI) therapy (including imatinib, and the second‐generation TKIs, dasatinib, nilotinib, and bosutinib).2 The prevalence of CML has risen steadily due to the substantial prolongation of survival achieved with TKI therapy, and is projected to continue to increase for the next several years.3 The life expectancy of patients with CML with optimal responses to TKI treatment is now approaching that of the general population, particularly in patients as young as 55 years.4
Imatinib has profoundly impacted the management of CML, allowing a substantial proportion of patients to achieve deep molecular response (DMR) on long‐term therapy.5 In a recent update of the CML‐IV study, the cumulative incidence of molecular response of a 4.5‐log reduction in BCR‐ABL1 transcripts (MR4.5) was approximately 50% after 5 years, increasing to 70% after 9 years.6 However, clinical trial data with second‐generation TKIs demonstrate higher rates of response compared with imatinib, with responses being deeper and occurring earlier. The final analysis of the 5‐year Dasatinib versus Imatinib Study in Treatment‐Naïve Chronic Myeloid Leukemia Patients Trial (DASISION) showed that 42% of patients who received first‐line dasatinib (vs 33% who received imatinib) achieved MR4.5 by 5 years.7 The Evaluating Nilotinib Efficacy and Safety in Clinical Trials–Newly Diagnosed Patients (ENESTnd) study comparing first‐line nilotinib with imatinib showed that 55% of patients who received nilotinib achieved MR4.5 by 6 years, compared with 45% who received imatinib.8
Despite these favorable data, long‐term use of second‐generation TKIs has been associated with adverse events such as pleural effusion and cardiovascular events,8, 9 which may increase disease morbidity or mortality. Moreover, with long‐term use, even less severe adverse events such as fatigue or musculoskeletal pain may affect patients' quality of life (QoL).10 Finally, high up‐front costs of TKI treatment can be daunting to healthcare systems, and these costs are associated with nonadherence in patients who are responsible for a large co‐payment. In one study of patients who had relatively high cost‐sharing, 42% were less likely to adequately adhere to treatment.11 Nonadherence for any reason was also associated with poor responses.
Because life expectancies for patients treated with TKIs are approaching that of the general population,12 and sustained DMR can be achieved in a significant number of patients,7, 8 treatment‐free remission (TFR) has become a goal for many patients with CML. In this review, we discuss recent literature and current trials evaluating TFR in patients with CML treated with TKIs, in order to put current knowledge about the feasibility of choosing to stop TKIs into context. We also examine the patient factors to be assessed when deciding to implement TFR as a treatment goal for a patient with CML, including which patients can be considered for attempting TFR, when TKI discontinuation may be implemented, and, importantly, what the current recommendations are for proper monitoring. Additionally, unanswered questions and future prospects will be discussed.
2. KEY CONSIDERATIONS PRIOR TO STOPPING TKI TREATMENT
2.1. When to stop TKI treatment
Duration and response to treatment (including both the depth and duration of response) before discontinuing have factored into decisions regarding treatment cessation. Essential for consideration of elective treatment discontinuation is achievement of DMR that is sustained for a minimum period. Several discontinuation trials established sustained MR4 for at least 2 years as the fundamental criterion for considering treatment discontinuation,13 although the specific eligibility criteria vary across TFR trials.
In some patients, treatment with TKIs for an extended period has led to a more successful TKI discontinuation. In the Stop Imatinib (STIM) trial of imatinib discontinuation, long‐term follow‐up showed that patients with CML who received imatinib for ≥50 months experienced fewer molecular relapses than those who received imatinib for less than 50 months.14, 15 Achievement of an early molecular response recently has been proposed as a predictive factor for successful treatment discontinuation.16
The depth of molecular response achieved prior to discontinuing may also be predictive of successful discontinuation. A single‐institution study of patient‐driven TKI discontinuation showed that stopping TKIs in patients with less than MR4.5 may lead to a sustained complete molecular response (CMR; with 5‐log sensitivity) in some patients, but the risk of molecular relapse was higher if patients were not in CMR at the time they discontinued treatment.17 Data from the ENESTfreedom (Nilotinib Treatment‐free Remission Study in CML Patients) and ENESTop (Treatment‐free Remission After Achieving Sustained MR4.5 on Nilotinib) trials suggest that some patients can achieve durable TFR with unstable CMR prior to treatment discontinuation, as TFR rates were similar for patients with and without sustained responses. However, these data are limited by the relatively small patient populations and short follow‐up times in both trials.18, 19 Although closely linked, the duration of DMR may be a more important factor than the duration of therapy. In the European Stop Tyrosine Kinase Inhibitor Trial (EURO‐SKI) trial, prognostic modeling suggested TFR success is more dependent upon the duration of DMR (defined as MR,4 or a 4‐log reduction in BCR‐ABL1 transcripts) than the time on therapy before achievement of MR,4 indicating that duration of response was the most relevant factor.20 Another analysis suggested that patients with sustained MR4.5 for ≥5 years have a 15% chance of molecular relapse after treatment discontinuation.21
An important factor to consider is the stage and course of the disease. In the Stop Second‐Generation (STOP‐2G‐TKI) study, patients receiving first‐line TKIs and patients discontinuing their first‐line TKI due to intolerance had a higher probability of success than patients previously experiencing resistance to their first‐line TKI.22 Data regarding TFR for patients who have previously progressed to accelerated or blast phase are limited, but the consensus is that such patients should not attempt treatment discontinuation outside of a clinical trial.
Sokal risk score at the time of diagnosis has been identified as an important prognostic factor for successful TFR with imatinib.23, 24 Data from the CML8 (TWISTER) study of the Australasian Leukemia and Lymphoma Group (ALLG) of patients with CML who discontinued imatinib treatment suggested that a high‐risk Sokal score at diagnosis was associated with molecular relapse.23 Mahon et al. reported that patients from STIM with low‐risk Sokal scores had an estimated survival without relapse at 18 months of 54%, compared with 35% and 13% in those with intermediate and high scores, respectively.14 Age and previous interferon treatment also have been suggested as potential prognostic factors for molecular relapse‐free survival.25, 26
In a recent survey given to practicing oncologists and hematologists prior to the publication of new practice guidelines, greater than 30% of physicians (102 of 300) reported attempting TKI discontinuation.27 Among these, 76% would consider stopping treatment because of adverse events, while fewer would consider TFR for economic reasons (35%) or adequate responses achieved (12%). However, the conditions under which TKI therapy was discontinued differed from current practice guidelines, which may have resulted in improper monitoring as well as higher rates of unsuccessful TFR attempts. Regional and institutional standards and recommendations for patient criteria before discontinuing TKIs have been proposed to help guide clinicians in safely and properly offering this option to selected patients.28 These include the availability of rapid, sensitive monitoring techniques (discussed in detail below), and structured patient follow‐up if BCR‐ABL1 levels should rise, so that patients can restart treatment rapidly. Figure 1 contains some of the relevant criteria to take into account when considering TFR as a treatment goal.
Figure 1.
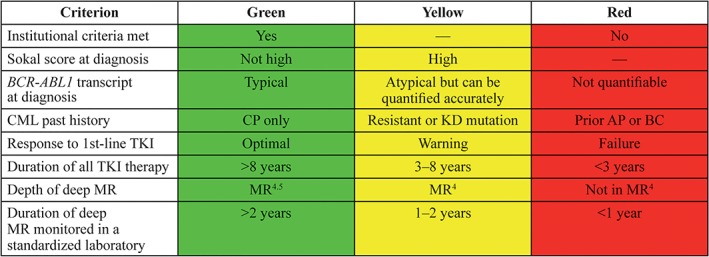
Possible criteria for selection of TFR as a treatment option. All criteria green indicates strongly recommended. Any yellow criteria indicates only consider TFR in high‐priority circumstances (e.g. significant toxicity or planned pregnancy). Any red criteria indicates TFR not recommended except in a clinical trial. AP, advanced phase; BP, blast crisis; CML, chronic myeloid leukemia; CP, chronic phase; KD, kinase domain; MR, molecular response; TFR, treatment‐free remission; TKI, tyrosine kinase inhibitor. (Republished with permission of the American Society of Hematology, from Hughes TP, Ross DM. Moving treatment‐free remission into mainstream clinical practice in CML. Blood. 2016;128(1):17‐23. © 2016 by The American Society of Hematology. Permission conveyed through Copyright Clearance Center, Inc.)
2.2. Why patients may or may not consider TFR
Several reasons might motivate patients to cease TKI therapy. Importantly, improving their QoL may provide sufficient rationale for TFR consideration. Younger patients may be prompted by a desire to lessen the potential for future adverse events, or by personal/family goals, whereas older patients may seek to mitigate the adverse events they currently experience on TKI therapy. Some patients may consider the cost of treatment over their lifetimes as motivation for stopping treatment.
Because the life expectancy of patients with CML who achieve optimal responses to TKIs is approaching that of healthy individuals, an important aspect of CML management should include a focus on QoL concerns. Patients with CML reported poorer QoL compared with healthy individuals, particularly if the patients were younger and if they were female.10 As part of the ENESTfreedom trial, patients completed questionnaires that addressed their QoL while discontinuing first‐line nilotinib. Interestingly, similar levels of anxiety and depression were reported before and after treatment discontinuation.29 The ENESTop study examined TFR in patients who switched from imatinib to nilotinib.30, 31 As part of the study, questionnaires assessing patient QoL were given during the consolidation and TFR phases; changes in QoL between the times of assessment were minimal. The authors point out that these patients had relatively high QoL at the beginning of the study.30
A major concern in treating pediatric patients with CML is the safety of prolonged TKI administration. As such, this is a population in which there may be particular interest in stopping treatment, but data in this setting are considerably limited. Successful TFR in a small number of pediatric patients has been reported.32, 33 It should be noted that cessation of treatment in children is somewhat controversial, as it has been suggested that the disease may be more aggressive in this patient population; moreover, the same prognostic factors for adults may not also apply to children.34 However, this claim has not been confirmed.
Female patients of childbearing potential may particularly benefit from TFR as a treatment goal, because the use of TKIs during pregnancy has been associated with adverse outcomes.35, 36, 37 Preclinical studies and a review of clinical cases have shown that dasatinib interfered with normal pregnancy, resulting in fetal abnormalities.37 Although anecdotal reports have cited instances of patients with uneventful pregnancies despite continuous administration of TKIs, this practice currently is not recommended.38
Adverse events with TKIs have been well documented, and the possibility of mitigating them may persuade some patients to pursue TFR. Notable adverse events associated with dasatinib are increased pulmonary arterial hypertension (PAH) and pleural effusion.9, 39 Additionally, increased peripheral arterial occlusive disease has been observed with several TKIs.40 However, it is important to carefully discuss all adverse events the patient is experiencing, in order to properly manage expectations. Some adverse events may be coincidentally present during TKI therapy, but not caused wholly or partially by a TKI. In other instances, symptoms may occur as a consequence of TKI withdrawal.28, 41, 42 The incidence of withdrawal syndrome is also variable across TFR studies. For example, results from The Dasatinib Discontinuation in Patients with Chronic‐Phase Chronic Myeloid Leukemia and Stable Deep Molecular Response (DASFREE) study reported a low incidence of withdrawal symptoms.43 To what extent the occurrence of withdrawal syndrome is affected by a specific TKI or reflective of varying trial criteria and reporting has yet to be determined. Recent analyses of studies of nilotinib discontinuation have documented no improvement in pain‐reported outcomes after treatment discontinuation.44 These reasons alone are insufficient to avoid treatment discontinuation, as there are other potential benefits, but assisting patients in a realistic understanding of treatment outcomes may help avoid frustration in the event that little to no improvement in certain symptoms occurs.
Economic considerations may play a key role in a patient's decision to attempt TFR. Along with new treatment choices for CML have come higher prices. In a study of data derived from the ENESTnd trial of nilotinib in patients newly diagnosed with CML, there was an incremental cost‐effectiveness ratio of €39 935 per quality‐adjusted life‐year for patients receiving nilotinib—a cost that could, at least in part, be offset by TFR.45 Another study showed that 70% of patients with high cost‐sharing requirements were likely to discontinue TKI treatment, frequently on their own, and 42% were likely to be nonadherent to therapy.11 Ideally, patients should not be motivated to opt for TFR by economic factors, but in the current environment, this is a consideration for many patients and third‐party payers. Having data to support their choice would benefit patients and may improve clinical outcomes.
It should be noted that many patients are unwilling to halt treatment. In one survey, only 42% of respondents were willing to stop TKI treatment due to fears of disease recurrence and increased mortality.46 In this single‐institution study, 26%‐62% of patients were willing to accept discontinuation of their TKI, depending upon the perceived success rate of cessation, and the risks of disease recurrence and increased mortality. Moreover, the decision to cease therapy may not always be a rational one: Riva et al found that both emotional and cognitive components influenced the decision to attempt TFR.47 They concluded that psychological reasons underlying the decision to halt treatment should be taken into consideration. Undoubtedly, this is a decision that is modulated by many factors, and one where patients' wishes and fears must be considered and respected. However, patients currently taking TKIs who have met the criteria for TFR may be unwilling to consider treatment discontinuation based on the education and counseling provided to them at the time treatment was initiated. Until recently, healthcare providers have counseled their patients that the duration of treatment would likely be for the patient's lifetime, and emphasis was placed on the negative impact any missed doses would have on the long‐term clinical outcome. As such, this notion may be deeply rooted in the current fears of the patient. It is important that the possibility of TFR be introduced as new patients begin TKI therapy, with cautious discussion of all its caveats and precautions. The potential for TFR should be introduced in such a way that it can motivate adherence and provide realistic goals when circumstances permit.
3. OUTCOMES WITH FIRST‐ AND SECOND‐GENERATION TKI DISCONTINUATION
The pioneering TFR trials with imatinib established the feasibility and practicality of TKI discontinuation, and, to date, the majority of data regarding TFR are based on patients treated with imatinib.14, 15, 23, 41 However, TFR data with second‐generation TKIs are emerging, and several of these discontinuation trials are currently ongoing. Clinical trial data regarding dasatinib and nilotinib discontinuation, including key elements of each study, TFR eligibility, line of therapy, and relapse rate, are shown in Table 1.22, 31, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70
Table 1.
Clinical trials of TFR with second‐generation TKIs
| Registry information | Trial title | Enrolled patients/line of therapy | TFR eligibility | Relapse definition | TFR rate; time after discontinuing | Patients regaining molecular response after reinitiating TKIs | References |
|---|---|---|---|---|---|---|---|
| NCT0185004 | Open‐label study evaluating Dasatinib therapy discontinuation in patients with chronic phase CML with stable complete molecular response (DASFREE) | 84/dasatinib (frontline or subsequent lines) | MR4.5 For ≥1 year | Loss of MMR | 48%; 1 year | 42/42a | Shah et al.38 |
| NCT01627132 | Discontinuation of Dasatinib in patients with chronic myeloid leukemia‐CP who have maintained complete molecular remission for 2 years; Dasatinib stop trial (D‐STOP) | 65/dasatinib (frontline or subsequent line after imatinib) | MR4 for 2 years | 2 positive MR4 readings in 1 month | 63%; 1 year | 20/20 | Kumagai et al.57 |
| NCT02268370 | Treatment‐free remission accomplished with Dasatinib in patients with CML (TRAD) | 118/dasatinib rechallenge and discontinuation after imatinib discontinuation (second‐stop) | MR4.5 For ≥2 years | Loss of MR4 on 2 consecutive occasions or MMR on 1 occasion | 67%; 1 year | 35/40 | Kim et al.59 |
| NCT01761890 | Front‐line treatment of BCR‐ABL+ chronic myeloid leukemia (CML) with Dasatinib (CML1113) | 133/dasatinib (frontline) | CMR | NR | NR | NR | CML111343 |
| UMIN000005130 | Discontinuation of Dasatinib in patients with chronic myeloid leukemia‐CP who have maintained complete molecular remission for 2 years; Dasatinib stop trial (DADI) | 63/dasatinib (frontline or subsequent line after imatinib) |
MR4 for ≥1 year | Loss of MR4 | 44%; 3 years | NR | Okada et al.58
DADI44 |
| UMIN000008999 | Dasatinib discontinuation for CML‐CP with 1 year CMR (IMIDAS) | 50/dasatinib | CMR for ≥1 year | NR | NR | NR | IMIDAS45 |
| UMIN000011099 | DAsatinib DIscontinuation for 1st‐line treatment with CML‐CP with 1 year CMR (1st DADI) | 100/dasatinib (frontline) | CMR for ≥1 year | Loss of CMR | NR | NR | 1st DADI46 |
| NCT01887561 | Dasatinib for patients achieving complete molecular response for cure (D‐NewS) | 100/dasatinib (frontline) | NR | NR | NR | NR | D‐NewS47 |
| UMIN000008583 | Clinical research of safety and efficacy after a stop of dasatinib administration in patients with CML‐CP achieving a CMR on a treatment of TKIs (STDAST) | 60/imatinib, nilotinib + dasatinib, dasatinib | CMR for ≥2 years | NR | NR | NR | STDAST48 |
| UMIN000007221 | Multicenter clinical study evaluating the safety and efficacy of Dasatinib treatment and its discontinuation in patients with CP CML who had reached CMR during Imatinib therapy | 40/dasatinib (after imatinib) | CMR on imatinib | NR | NR | NR | Multicenter clinical study on the safety and efficacy of Dasatinib Discontinuation49 |
| UMIN000022254 | Phase II clinical trial of Dasatinib therapy aiming for TFR in patients with CP‐CML (D‐FREE) | 300/dasatinib (frontline) | MR4.5 For ≥1 year | Loss of MMR once, or loss of MR4 twice | NR | NR | D‐FREE50 |
| UMIN000007141 | Multicenter phase II clinical study of the safety and efficacy of discontinuing Nilotinib treatment in patients with chronic phase chronic myelogenous leukemia who have achieved complete molecular response with Imatinib or Nilotinib (NILSt) | 112/nilotinib (frontline, or after imatinib) | MR4.5 For 2 years | Loss of MR4.5 | 59%; 1 year | 32/34 | Kadowaki et al.39 |
| NCT01784068 | Nilotinib treatment‐free remission study in CML patients (ENESTfreedom) | 215/nilotinib (frontline) | MR4.5 | Loss of MMR | 49%; 96 weeks | 87/88b | Hochhaus et al.60
Ross et al.61 |
| NCT01744665 | Safety and efficacy of CML patients who switch to Nilotinib and stop treatment after achieving and sustaining MR4.5 (ENESTGoal) | 59/nilotinib (after imatinib) | MR4.5 | Loss of MMR | 41%; NR | NR | Ritchie et al.62 |
| NCT01698905 | Treatment‐free remission after achieving sustained MR4.5 On Nilotinib (ENESTop) | 163/nilotinib (after imatinib) | MR4.5 For ≥2 years on nilotinib | Loss of MMR or confirmed loss of MR4 | 53%; 96 weeks | 54/56 | Mahon et al.25 |
| UMIN000005904 | Multicenter clinical study on the safety and efficacy of Nilotinib discontinuation in patients with chronic myelogenous leukemia‐chronic phase and complete molecular response (STAT2) | 96/nilotinib (after imatinib) | MR4.5 for 2 years | Loss of MR4.5 | 68%; 1 year | NR | Takahashi et al.63
Stop Tasigna Trial51 |
| NCT01743989 | A randomized phase III study to assess the effect of a longer duration of consolidation treatment with Nilotinib on TFR in CP CML (ENESTPath) | 619/nilotinib (after imatinib) | Stable MR4 for ≥1 year | Loss of MR4 | NR | NR | ENESTPath52 |
| NCT01657604 | Tasigna and interferon alpha evaluation initiated by the German CML study group (TIGER) | 652/nilotinib, nilotinib+IFN (frontline) | Confirmed MMR for ≥2 years | Loss of MMR | NR | NR | TIGER53 |
| NCT02917720 | Stop second generation TKI study (STOP‐2G TKI) | 100/dasatinib or nilotinib (frontline or subsequent line) | MR4.5 For ≥2 years | Loss of MMR | 63%; 1 year | 26/26 | Rea et al.20 |
| NCT01596114 | European stop tyrosine kinase inhibitory study (EURO‐SKI) | 821/imatinib, dasatinib, or nilotinib (frontline or second line due to intolerance) | MR4 for ≥1 year | Loss of MMR | 52%; 2 years | 86% | Mahon et al.65 |
| NCT02269267 | The life after stopping tyrosine kinase inhibitors study (the LAST study) | 173/imatinib, dasatinib, nilotinib, or bosutinib | MR4 for ≥2 years | Molecular recurrence | 66%; 12.3 months | 48 | LAST54
Atallah et al.65 |
| NCT01804985 | De‐escalation and stopping treatment of Imatinib, Nilotinib or sprYcel in chronic myeloid leukemia (DESTINY) | 174/frontline imatinib, dasatinib, or nilotinib (second line if due to intolerance allowed) | MMR for 1 year | Loss of MMR in 2 consecutive samples | 93%; 1 year | 12/12 | Clark et al.55 |
| UMIN000007944 | Stop imatinib or dasatinib study in CP‐CML patients maintaining CMR for 2 years (TWMU‐TKI‐STOP) | 40/imatinib or dasatinib | CMR for 2 years | NR | NR | NR | TWMU‐TKI‐STOP (2012)56 |
Abbreviations: CCyR, complete cytogenetic response; CML‐CP, chronic myeloid leukemia in chronic phase (also called CP CML in some trials); CMR, complete molecular response; IFN, interferon; MMR, major molecular response; MR4, molecular response of 4‐log reduction of BCR‐ABL1 transcripts; MR4.5, molecular response of 4.5‐log reduction of BCR‐ABL1 transcripts; MRFS, molecular recurrence‐free survival; TFR, treatment‐free remission; TKI, tyrosine kinase inhibitor.
One patient lost MMR and restarted treatment. The patient was not evaluable because he/she discontinued the study after only one PCR assessment.
One patient who did not regain MMR after restarting nilotinib withdrew consent and discontinued after 7.1 weeks of treatment, and was not eligible for evaluation.
3.1. Imatinib
Results from several long‐term imatinib discontinuation trials, including STIM, have shown that approximately half of the patients with sustained DMR can successfully maintain their responses after entering TFR.1, 14, 15, 23, 41 STIM first selected patients with undetectable transcripts (with a sensitivity of real‐time quantitative polymerase chain reaction [RQ‐PCR] of at least 5 logs) sustained for more than 2 consecutive years, and with at least five assessments during those 2 years (ie, an assessment every 6 months).14 Because of the difficulties of reaching this level of sensitivity, with MR4.5 being a more standardized goal, subsequent studies have focused on this level of sustained response to consider attempts at TFR.23, 71 The initial STIM trial also considered relapse as the reappearance of detectable transcripts.14 However, with longer follow‐up it became clear that some patients may experience reappearance of detectable transcripts at low levels with no further increase (and in some instance spontaneous decrease to below levels of detection). Therefore, the A‐STIM study proposed changing the criterion for treatment resumption to loss of MMR.26 Generally, all patients who relapsed in imatinib discontinuation trials remained sensitive to TKIs and regained molecular response upon re‐treatment. Depending on the specific definition of relapse, approximately half of patients experienced relapse within the first 6‐12 months after stopping imatinib treatment, and the remaining patients (40%‐60%) have maintained their responses throughout the duration of follow‐up.1, 14, 15, 23, 41 Among the predictive factors associated with improved probability of successful imatinib discontinuation are lower Sokal risk score, prior use of interferon, longer duration of therapy with imatinib, and longer duration of molecular response.14, 20, 26 Patients with successful TFR following imatinib discontinuation were also identified as having an increase in natural killer cells compared with patients who relapsed, suggesting some role of immune mechanisms in maintaining response after discontinuation, despite the known inability of TKIs to eradicate the earliest leukemic progenitors.72 Imatinib discontinuation studies also identified the occurrence of what is now termed a withdrawal syndrome, characterized by musculoskeletal pain that frequently improves spontaneously or with anti‐inflammatory agents but occasionally (albeit rarely) may require resumption of TKI therapy.28, 42
3.2. Dasatinib
The DASFREE study examined discontinuation of dasatinib in the first and subsequent lines in patients who maintained DMR (defined as MR4.5) for ≥1 year.43 Patients in DASFREE (N = 84) had a high TFR success rate (48% had MMR, and 49% had event‐free survival) 1 year after discontinuing. Reassuringly, all evaluable patients who relapsed regained MMR quickly after reinitiating treatment. The Discontinuation of Dasatinib in Patients With Chronic Myeloid Leukemia‐CP Who Have Maintained Complete Molecular Remission for 2 years (D‐STOP) trial evaluated TFR in patients on dasatinib who maintained DMR (defined as MR4) for ≥2 years.62 At 1 year, the estimated TFR rate was 63%, and all relapsed patients responded to dasatinib reinitiation. In the Dasatinib Discontinuation for Chronic Myelogenous Leukemia‐Chronic Phase with One Year Complete Molecular Remission (DADI) trial, which assessed TFR in patients who maintained DMR ≥1 year prior to discontinuing dasatinib, the estimated overall TFR was 44% at 3 years.63 The Treatment‐free Remission Accomplished With Dasatinib in Patients With CML (TRAD) study is currently evaluating whether dasatinib treatment after failure of TFR with imatinib would lead to a successful TFR attempt.64 Eligible patients in TRAD had achieved MR4.5 ≥2 years prior to TFR enrollment. In an interim analysis, the TFR rate following discontinuation of imatinib was 67% at 1 year. All patients who received dasatinib therapy because of relapse regained a response. Among these patients, seven achieved sustained MR4.5 over 1 year and have discontinued treatment for the second time. Currently, five of these patients remain in TFR and two have lost MMR.
3.3. Nilotinib
There are published outcomes from several trials examining TFR following nilotinib treatment. The STOP NILOTINIB (NILSt) trial was a single‐arm trial in Japan of patients who achieved MR4.5 with either imatinib or nilotinib, then were treated for additional years with nilotinib.44 In all, 87 patients maintained MR4.5 and were eligible to stop nilotinib, and at 1 year, 53 (58.9%) patients maintained their response. Of 34 patients who lost MR4.5, 32 regained it after restarting nilotinib. The ENESTfreedom study reported that of the 190 patients with sustained DMR (MR4) that attempted TFR, 93 (49%) remained off treatment without loss of MMR at 96 weeks.65, 66 Of the 88 patients who restarted treatment following loss of MMR, 87 regained MMR and 81 regained MR4.5 by the data cutoff. ENESTgoal (Safety and Efficacy of CML Patients Who Switch to Nilotinib and Stop Treatment After Achieving and Sustaining MR4.5) is evaluating the rate of TFR in patients who have achieved sustained DMR (MR4.5) with second‐line nilotinib after switching from imatinib.67 Although only a small number of patients (N = 17) have entered the TFR portion of this study thus far, seven (41%) currently remain in TFR. One patient discontinued from the study while in TFR, and nine experienced molecular relapse and have since entered the reinitiation phase. In ENESTop, a trial evaluating TFR in patients achieving MR4.5 on nilotinib for ≥2 years after prior therapy with imatinib, 58% (73/126) of patients remained in molecular remission at 48 weeks.31 A subgroup analysis of ENESTop, based on reasons for switching to nilotinib prior to TFR, showed that of 125 patients included in this analysis, 51 switched due to intolerance, 30 switched due to resistance, and 44 switched due to physician preference.73 The proportion of those who maintained TFR at 48 weeks after discontinuing nilotinib was similar across groups: 30 (58.8%) in the intolerance subgroup, 16 (53.3%) in the resistance subgroup, and 27 (61.4%) in the physician preference subgroup. In the Multicenter Clinical Study on the Safety and Efficacy of Nilotinib Discontinuation in Patients with Chronic Myelogenous Leukemia‐Chronic Phase and Complete Molecular Response (STAT2) trial, which evaluated TFR in patients who had achieved MR4.5 with nilotinib, 52 of 75 (68%) patients entering TFR remained without molecular reoccurrence after 1 year.68
3.4. Multiple TKIs
The STOP‐2G‐TKI study assessed the duration of MMR following treatment discontinuation in patients who received second‐generation TKIs (dasatinib or nilotinib), and who achieved MR4.5 for ≥2 years.22 Cumulative incidences of molecular relapse by 12 and 48 months were 35% and 45%, respectively. TFR rates at 12 and 48 months were 63% and 54%, respectively. In univariate analysis of STOP‐2G‐TKI, prior suboptimal response or TKI resistance was the only baseline risk factor associated with decreased TFR. During the treatment‐free phase, no progression to advanced‐phase CML occurred, and all relapsing patients regained MMR and MR4.5 after restarting therapy. In the EURO‐SKI study, patients treated with imatinib, nilotinib, or dasatinib who had no treatment failure were eligible if they maintained MR4 for ≥1 year. In 755 eligible patients, the molecular recurrence‐free survival rate was 50% at 24 months.69 In the U.S. Life after Stopping TKIs (LAST) study, 66% (115/173) of all patients discontinuing imatinib, dasatinib, nilotinib, or bosutinib remained in TFR after a median follow‐up of 12.3 months.70 Lastly, in an observational study of 293 Italian patients with chronic‐phase CML discontinuing TKIs in first‐line (55%) and subsequent‐line (45%) settings, at 1 year the estimated TFR was 68% for imatinib and 73% for second‐generation TKIs (dasatinib, nilotinib, or bosutinib).74
3.5. Real‐world TKI discontinuation
Results for stopping TKIs outside of clinical trials have been published. A retrospective analysis of unplanned TKI discontinuations revealed that seven of the 13 patients who discontinued treatment remained in complete molecular response (CMR; defined as no detection of BCR‐ABL1 transcripts), with a median follow‐up of 26 months.75 Of the remaining six patients who lost CMR following TKI discontinuation, two achieved a second CMR following readministration of TKIs, two spontaneously achieved CMR without TKI treatment, and two remained in complete hematological response without further therapy, with a median follow‐up time of 29.5 months. The molecular relapse profiles were compared in another study of patients who discontinued imatinib, nilotinib, or dasatinib after sustaining undetectable BCR‐ABL1 for ≥2 years. Relapses occurred after median intervals of 4 months for imatinib and dasatinib and 5 months for nilotinib, and the different kinetics indicate that the BCR‐ABL1+ cells active in relapse for different TKIs may vary as well.76 The outcomes of patients choosing to discontinue TKI treatment regardless of their ongoing response have also been evaluated in a single‐institution study.17 Of the 27 patients with CML who discontinued treatment with CMR, 11 (41%) had a molecular relapse after a median of 3.5 months.
4. ONGOING TRIALS OF TKI DISCONTINUATION
Many additional trials are currently investigating outcomes for TFR as a treatment goal in CML. When sustained MR4.5 for ≥2 years is considered the criterion for TFR eligibility, approximately 40%‐45% of patients treated with nilotinib and 20%‐25% of patients treated with imatinib would be eligible for TFR.14, 25, 77 Some of the questions these studies are attempting to address are whether TFR can be accomplished successfully with responses less than MR4.5, or responses that have been sustained for less than 2 years. Lowering these standards may increase the pool of eligible patients, although the increase in eligibility should be considered only in the context of possible increases in relapse rates. Until this balance is better defined, these approaches should not be considered outside the context of clinical trials. Some relevant studies are described in Table 1.22, 31, 43, 44, 67, 68, 69, 70
5. MOLECULAR MONITORING
Accurate, sensitive molecular monitoring of BCR‐ABL1 transcript levels is essential before deciding on TFR, in order to identify patients with sufficiently deep molecular responses, as well as for the continued monitoring of the disease state once treatment has stopped. Importantly, accurate monitoring allows early detection of patients who relapse, so that TKIs can be restarted immediately.
Recommendations for molecular monitoring during treatment, as well as during the treatment‐free period, are evolving. The National Comprehensive Cancer Network,72 the European LeukemiaNet,78 and the European Society for Medical Oncology (ESMO)3 have issued guidance on monitoring responses to TKIs. All agencies recommend monitoring BCR‐ABL1 levels before and during TKI treatment; however, the NCCN and ESMO have specific recommendations for monitoring BCR‐ABL1 during TFR. Minimal institutional requirements for TFR are also available, which may help guide the oncologist when considering TKI discontinuation for patients.28
5.1. Key considerations for monitoring
A major consideration is accessibility of suitable laboratories, as not every medical center has access to RQ‐PCR or digital PCR technology, the most sensitive and accurate methods for quantifying transcripts. There should be an accepted method for standardizing reporting of molecular responses. This is the one used by most locales today, but it still has not been adopted universally.79 Additionally, the laboratory must have adequate quality control mechanisms to ensure that the results provided are accurate and reproducible. Finally, current ESMO guidelines highlight the need for a rapid turnaround of PCR test results within 4 weeks, as well as capacity to provide PCR tests every 4‐6 weeks when required, both of which are feasible only in a suitably equipped laboratory.3
Accuracy of transcript quantification depends in part on the number of control gene transcripts analyzed per replicate in samples with either detectable or undetectable BCR‐ABL1 transcripts; a minimum of 10 000 BCR‐ABL1 and 24 000 GUSB transcripts have been suggested to assess MR4; for MR4.5, a minimum of 32 000 BCR‐ABL1 and 77 000 GUSB transcripts should be analyzed.80 Results from the EURO‐SKI trial, for example, demonstrate that detecting MR4.5 depends on detecting an adequate number of control gene transcripts, and that the higher the number, the greater the sensitivity (the greater the chance that BCR‐ABL1 transcripts will be detected).81
To accurately monitor patient status during a period of TFR, BCR‐ABL1 transcripts should be typical or otherwise easily quantified. Approximately 2%‐3% of newly diagnosed patients with CML express aberrant transcripts such that MR4 or MR4.5 cannot be readily determined.28 In these instances, fluorescent in situ hybridization (FISH) may be the most sensitive quantitative tool available. However, use of FISH is not currently advised in the consideration of TFR because it is several orders of magnitude less sensitive than PCR, and there is uncertainty about the depth of response that is detected.
Indisputable criteria for TFR considerations are proper monitoring before and after discontinuation. In the STIM study, for example, it was required that patients had at least five determinations confirming that DMR was sustained for ≥2 years, and they were properly monitored every 6 months while receiving imatinib. Recent studies have started to explore different criteria for treatment discontinuation such as MR4 sustained for only 1 year. The EURO‐SKI study (which included patients with any TKI, although a majority were treated with imatinib) used such criteria and reported a 49% probability of loss of MMR.69 However, because of the short follow‐up (median of 27 months) and what appears to be a slow but continued drop in the rate of successful TFR over time, such criteria cannot be considered standard at the time of this report.
A standardized schedule for monitoring should also be established once TFR begins. The more mature studies have used monthly monitoring for at least the first 6‐12 months, then every 2 months for 6‐12 months, and every 3‐6 months thereafter. In recent trials, monitoring was performed as infrequently as every 4 weeks initially, and after 6 months the interval was lengthened to every 3 months.25 Current NCCN guidelines suggest monthly monitoring for the first 12 months after discontinuation of TKI, every 6 weeks during months 13‐24, and every 12 weeks thereafter.13 Not performing proper monitoring after discontinuation puts the patient at risk of an unrecognized relapse that can turn an exciting opportunity for patients into a terrible situation. Whether monitoring can be suspended at some point in the long term remains a matter for research. Although the bulk of relapses occur within the first 6 months,25 experience with other modalities such as stem cell transplant tells us that few patients have very late relapses, even beyond 15 years after transplant.26 Considering the relatively low cost and minimal discomfort of continued monitoring, continued and indefinite monitoring is currently advisable. An important concern is patient compliance, as the patient has to be willing and able to commit to monitoring in accordance with the recommended schedule, and the physician must reciprocate with a commitment to perform the monitoring as recommended. A retrospective study to determine how compliant patients with CML are with regard to molecular monitoring showed that approximately 50% of patients adhered to the monitoring schedule,82 while a similar study of patients in Lebanon showed that only 42% adhered to monitoring requirements.83
The laboratory should have the capability to rapidly return results, ideally in less than 4 weeks. A structured protocol to follow up with patients if BCR‐ABL1 levels rise should be in place, including treatment details and monitoring frequency. If sensitive standardized PCR is not available, then TFR should not be pursued.
5.2. Changing technology and its use in monitoring molecular responses
RQ‐PCR has become an increasingly standard technique,79 yet still more sensitive techniques may be used in the future. Digital PCR is reported to be 100 times more sensitive than RQ‐PCR, which can detect a single BCR‐ABL1+ cell out of 107 cells.84, 85 In the ENESTgoal study, digital PCR was used to detect BCR‐ABL1 transcripts in some patients who had undetectable transcripts measured by RQ‐PCR.27 In another recent study, digital PCR and age predicted risk of relapse: 100% of patients who were aged less than 45 years and had a positive digital PCR relapsed, whereas only 31% of patients ≥45 years and who had negative digital PCR relapsed.85 Although this methodology is not currently available in most clinical laboratories, it will likely become more mainstream in the next few years.
6. WHEN TO RESTART TREATMENT FOLLOWING TKI DISCONTINUATION
Earlier studies used reappearance of detectable transcripts to define relapse.14, 23 From these studies, it became evident that while some patients show a steady increase of transcript levels after discontinuation, in many instances low transcript levels remained, often fluctuating between undetectable and low levels (ie, MR4.5). This has led to the notion that relapse, and thus reinitiating therapy, should be considered when MMR is lost in most instances. Additionally, most ongoing trials and real‐world strategies use this guideline when they consider restarting TKI therapy.25 The NCCN and ESMO guidelines also suggest restarting treatment when MMR is lost, and ESMO specifically recommends establishing a structured follow‐up to allow for rapid intervention if BCR‐ABL1 is rising.3, 13 Some studies, however, such as the one by Tsutsumi and colleagues, still prefer loss of CMR as their criterion for restarting TKI, with CMR defined as no detectable BCR‐ABL1 transcripts.75
7. DISCUSSION
The treatment of CML is evolving such that a proportion of patients successfully treated with a TKI might expect to attempt a TFR period28; greater than 2000 patients have entered TFR periods after achieving DMR on TKIs (MR4 or better).25 Current evidence shows that 40%‐60% of patients relapse while in TFR; however, all or nearly all regain response once TKI treatment is reinitiated.25 Importantly, early data indicate that discontinuing TKI a second time may be successful in selected patient populations.86
Safety considerations for TFR include lack of response upon reinitiation of TKI, disease progression during the TFR period (although never confirmed in a properly monitored study), and withdrawal syndrome. Perhaps the risk causing most concern is the potential lack of molecular response upon restarting TKI; however, data from both clinical trials and discontinuation studies outside of clinical trials show that in the overwhelming majority of patients, responses have been regained when treatment has been reinitiated.22, 26, 44 In some instances, BCR‐ABL1 transcripts may not become undetectable again if that was the criterion used for discontinuation, but transcript levels will typically decrease to MMR and, most frequently, MR4.5. A retrospective analysis at one institution showed that when 13 patients lost CMR after discontinuing TKI (at their request), two regained CMR upon reinitiation of TKI, two regained CMR without reinitiating therapy, and two remained in complete hematological response without further treatment.75
There is a finite risk of relapse when a patient is in TFR, although relapse has been defined differently as the development of discontinuation trials progresses, from loss of MR4.5 to loss of MMR. Depending upon the study design, the relapse rate at 6 months has ranged anywhere from 61% in the STIM trial11 and 49% in the EURO‐SKI trial.69 This risk is an important consideration in the patient's decision to attempt treatment discontinuation. In a recent survey of greater than 1100 patients with CML, 49% responded they would not discontinue treatment for fear of losing the response they had achieved at the time of the survey.87 In another survey of 210 patients, 58% responded they might not attempt TFR for fear of losing the response they had obtained.46 The risk of relapsing into advanced disease seems to be low based on the trial data gathered thus far. In the According to STIM (A‐STIM) trial, one patient experienced a lymphoid blast crisis 9 months after restarting imatinib while in MMR. The patient underwent further treatment, including allogeneic stem cell transplantation, and was still living at the most recent follow‐up.26 Thus, although an important consideration—and one that underscores the need for proper monitoring after discontinuation—with adequate follow‐up of 5‐6 years, this risk appears to be minimal.
One final safety consideration is withdrawal syndrome. Withdrawal syndrome follows cessation of TKI therapy and consists of diffuse myalgia, arthralgia, or musculoskeletal pain, all of which respond to steroids or other analgesics.28, 41, 42 In the DASFREE study, musculoskeletal events were reported in 23 (27%) patients off treatment, although only eight events were attributed to TKI withdrawal.43 In the ENESTop trial, there was a higher incidence of musculoskeletal pain‐related adverse events observed during TFR (42% vs 14%).31 Nearly 10% of patients in the NILSt trial reported musculoskeletal pain.44 In a few instances, symptoms may be severe enough to warrant resuming therapy. A better understanding of the pathophysiology of this complication is needed in order to design strategies that mitigate symptoms and the need to resume therapy.
Unanswered questions remain. Can patients maintain long‐term responses in TFR or after reinitiating TKIs? Will the development of even more sensitive screening technologies push molecular responses to MR4.5 or better, and should patients need to meet these more stringent criteria before discontinuing TKIs? Will other predictors of successful discontinuation be identified? Can a consensus be reached as to the duration of DMR before recommending TFR? It is hoped that data from ongoing trials will help answer most, if not all, of these important questions.
Notably, 40% of patients treated met the prespecified criteria for TFR, based on the need for an MR4.5 sustained for ≥2 years.25 Among these patients, approximately 50% maintained their responses, meaning that only 20% had successful TFR. Approaches to improve both ratios are necessary in order to make TFR a more palpable reality for patients. Other strategies that are needed include improving the ratio of sustained molecular responses and/or decreasing the risk of relapse as needed. Considering that TKIs are not capable of eradicating the leukemic stem cell, several approaches are being considered both clinically and preclinically in order to achieve this endpoint, which may improve the ultimate goal of increasing the success rate of TFR.
In conclusion, the faster and deeper molecular responses observed with second‐generation TKIs may increase the number of patients with CML eligible for TFR. There are numerous patient factors that may affect the outcome of TFR, and patients must be willing and able to comply with the requirement for frequent monitoring. Accurate, sensitive, rapid, and available monitoring laboratories and adequate timing of monitoring are essential for successful TFR. On the physician side, a comprehensive understanding of the criteria for stopping, monitoring, and reinitiating treatment is necessary. Further studies are needed, especially to help define the best predictive factors for identifying the most appropriate patients.
CONFLICT OF INTEREST
JC has served as a consultant for and received research funding from ARIAD, Bristol‐Myers Squibb, Novartis, Pfizer, and Teva. DR has served as a consultant to Bristol‐Myers Squibb and Novartis, and received honoraria from Bristol‐Myers Squibb, Incyte, Novartis, and Pfizer. JHL has served as a consultant to, received research funding from, or served as an advisor to ARIAD, Bristol‐Myers Squibb, Novartis, and Pfizer.
AUTHOR CONTRIBUTIONS
The authors take full responsibility for the content of this publication and confirm that it reflects their viewpoints and medical expertise.
DATA‐SHARING STATEMENT
Bristol‐Myers Squibb (BMS) policy on data sharing may be found at: https://www.bms.com/researchers-and-partners/independent-research/data-sharing-request-process.html).
ACKNOWLEDGMENTS
They also wish to acknowledge Jessica Franciosi, PhD, and Andrea Lockett of StemScientific, an Ashfield company, part of UDG Healthcare plc, funded by Bristol‐Myers Squibb, for providing medical writing and editorial support.
Cortes J, Rea D, Lipton JH. Treatment‐free remission with first‐ and second‐generation tyrosine kinase inhibitors. Am J Hematol. 2019;94:346–357. 10.1002/ajh.25342
Funding information Bristol‐Myers Squibb
REFERENCES
- 1. Kantarjian HM, O'Brien S, Cortes JE, et al. Complete cytogenetic and molecular responses to interferon‐alpha‐based therapy for chronic myelogenous leukemia are associated with excellent long‐term prognosis. Cancer. 2003;97(4):1033‐1041. [DOI] [PubMed] [Google Scholar]
- 2. Talpaz M, Shah NP, Kantarjian H, et al. Dasatinib inimatinib‐resistant philadelphia chromosome–positive leukemias. N Engl J Med. 2006;354(24):2531‐2541. [DOI] [PubMed] [Google Scholar]
- 3. Hochhaus A, Saussele S, Rosti G, et al. The ESMO guidelines committee. Chronic myeloid leukaemia: ESMO clinical practice guidelines for diagnosis, treatment and follow‐up. Ann Oncol. 2017;28(Suppl 4):iv41‐iv51. [DOI] [PubMed] [Google Scholar]
- 4. Bower H, Björkholm M, Dickman PW, Höglund M, Lambert PC, Andersson TML. Life expectancy of patients with chronic myeloid leukemia approaches the life expectancy of the general population. J Clin Oncol. 2016;34(24):2851‐2857. [DOI] [PubMed] [Google Scholar]
- 5. Hochhaus A, Larson RA, Guilhot F, et al. Long‐term outcomes of imatinib treatment for chronic myeloid leukemia. N Engl J Med. 2017;376(10):917‐927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Helhmann R, Müller MC, Lauseker M, et al. Deep molecular response is reached by the majority of patients treated with imatinib, predicts survival, and is achieved more quickly by optimized high‐dose imatinib: results from the randomized CML‐study IV. J Clin Oncol. 2014;32(5):415‐423. [DOI] [PubMed] [Google Scholar]
- 7. Cortes JE, Saglio G, Kantarjian HM, et al. Final 5‐year study results of DASISION: the Dasatinib versus Imatinib study in treatment‐Naïve chronic myeloid leukemia patients trial. J Clin Oncol. 2016;34(20):2333‐2340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hughes TP, Larson RA, Kim D‐W, et al. Efficacy and safety of nilotinib vs imatinib in patients with newly diagnosed chronic myeloid leukemia in chronic phase: 6‐year follow‐up of ENESTd. Haematologica. 2015;100(Suppl):228. [Google Scholar]
- 9. Cortes JE, Jimenez CA, Mauro MJ, Geyer A, Pinilla‐Ibarz J, Smith BD. Pleural effusion in dasatinib‐treated patients with chronic myeloid leukemia in chronic phase: identification and management. Clin Lymphoma Myeloma Leuk. 2017;17(2):78‐82. [DOI] [PubMed] [Google Scholar]
- 10. Efficace F, Baccarani M, Breccia M, et al. GIMEMA. Health‐related quality of life in chronic myeloid leukemia patients receiving long‐term therapy with imatinib compared with the general population. Blood. 2011;118(17):4554‐4560. [DOI] [PubMed] [Google Scholar]
- 11. Dusetzina SB, Winn AN, Abel GA, Huskamp HA, Keating NL. Cost sharing and adherence to tyrosine kinase inhibitors for patients with chronic myeloid leukemia. J Clin Oncol. 2014;32(4):306‐311. [DOI] [PubMed] [Google Scholar]
- 12. Sasaki K, Strom SS, O'Brien S, et al. Relative survival in patients with chronic‐phase chronic myeloid leukaemia in the tyrosine‐kinase inhibitor era: analysis of patient data from six prospective clinical trials. Lancet Haematol. 2015;2(5):e186‐e193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. National Comprehensive Cancer Network . NCCN Clinical Practice Guidelines in Oncology. Chronic Myelogenous Leukemia, Version 1.2019. Fort Washington, PA: National Comprehensive Cancer Network; 2018. [DOI] [PubMed] [Google Scholar]
- 14. Mahon FX, Réa D, Guilhot J, et al. Intergroupe Français des Leucémies Myéloïdes Chroniques. Discontinuation of imatinib in patients with chronic myeloid leukaemia who have maintained complete molecular remission for at least 2 years: the prospective, multicentre Stop Imatinib (STIM) trial. Lancet Oncol. 2010;11(11):1029‐1035. [DOI] [PubMed] [Google Scholar]
- 15. Etienne G, Guilhot J, Rea D, et al. Long‐term follow‐up of the French Stop Imatinib (STIM1) study in patients with chronic myeloid leukemia. J Clin Oncol. 2017;35(3):298‐305. [DOI] [PubMed] [Google Scholar]
- 16. Hjorth‐Hansen H, Stenke L, Söderlund S, et al. Dasatinib induces fast and deep responses in newly diagnosed chronic myeloid leukaemia patients in chronic phase: clinical results from a randomised phase‐2 study (NordCML006). Eur J Haematol. 2015;94(3):243‐250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Benjamini O, Kantarjian H, Rios MB, et al. Patient‐driven discontinuation of tyrosine kinase inhibitors: single institution experience. Leuk Lymphoma. 2014;55(12):2879‐2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hughes TP, Hochhaus A, Kim D‐W, et al. Treatment‐free remission (TFR) among patients (pts) with chronic myeloid leukemia in chronic phase (CML‐CP) not initially eligible for treatment discontinuation due to unstable deep molecular response (DMR): Enestfreedom and Enestop. Presented at the 59th Annual Meeting & Exposition of the American Society of Hematology, December 9‐12, 2017; Atlanta, Georgia. Abstract 2878.
- 19. Saglio G, Hughes T, Hochhaus A, et al. Impact of treatment cessation on overall disease outcomes in patients with chronic myeloid leukemia in chronic phase (CML‐CP) attempting treatment‐free remission (TFR): findings from Enestfreedom and Enestop. Blood. 2017;130(Suppl 1):1598. [Google Scholar]
- 20. Saussele S, Richter J, Guilhot JG, et al. Duration of deep molecular response has most impact on the success of cessation of tyrosine kinase inhibitor treatment in chronic myeloid leukemia—results from the EURO‐SKI trial. Blood. 2017;130(Suppl 1):313. [Google Scholar]
- 21. Chamoun K, Kantarjian HM, Rios MB, et al. CML patients outcome after TKI discontinuation: a single institution experience in the US. Blood. 2016;128:1923. [Google Scholar]
- 22. Rea D, Nicolini FE, Tulliez M, et al. France Intergroupe des Leucémies Myéloïdes Chroniques. Discontinuation of dasatinib or nilotinib in chronic myeloid leukemia: interim analysis of the STOP 2G‐TKI study. Blood. 2017;129(7):846‐854. [DOI] [PubMed] [Google Scholar]
- 23. Ross DM, Branford S, Seymour JF, et al. Safety and efficacy of imatinib cessation for CML patients with stable undetectable minimal residual disease: results from the TWISTER study. Blood. 2013;122(4):515‐522. [DOI] [PubMed] [Google Scholar]
- 24. Yhim HY, Lee NR, Song EK, et al. Imatinib mesylate discontinuation in patients with chronic myeloid leukemia who have received front‐line imatinib mesylate therapy and achieved complete molecular response. Leuk Res. 2012;36(6):689‐693. [DOI] [PubMed] [Google Scholar]
- 25. Saussele S, Richter J, Hochhaus A, Mahon FX. The concept of treatment‐free remission in chronic myeloid leukemia. Leukemia. 2016;30(8):1638‐1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rousselot P, Charbonnier A, Cony‐Makhoul P, et al. Loss of major molecular response as a trigger for restarting tyrosine kinase inhibitor therapy in patients with chronic‐phase chronic myelogenous leukemia who have stopped imatinib after durable undetectable disease. J Clin Oncol. 2014;32(5):424‐430. [DOI] [PubMed] [Google Scholar]
- 27. Ritchie EK, Wolff JE, Latremouille‐Viau D, et al. Tyrosine kinase inhibitor (TKI) therapy discontinuation in patients with CML in chronic phase: a US clinical practice perspective. J Clin Oncol. 2017;35(Suppl):7052. [Google Scholar]
- 28. Hughes TP, Ross DM. Moving treatment‐free remission into mainstream clinical practice in CML. Blood. 2016;128(1):17‐23. [DOI] [PubMed] [Google Scholar]
- 29. Hochhaus A, Gomez‐Cesares MT, Stentoft J, et al. Patient‐reported quality of life before and after stopping treatment in the ENESTfreedom trial of treatment‐free remission for patients with chronic myeloid leukemia in chronic phase. Blood. 2016;128:3066. [Google Scholar]
- 30. Mahon FX, Boquimpani C, Takahashi N, et al. Patient‐reported quality of life before and after stopping treatment in the ENESTop trial of treatment‐free remission for patients with chronic myeloid leukemia in chronic phase. Blood. 2016;128:1891. [Google Scholar]
- 31. Mahon FX, Boquimpani C, Kim DW, et al. Treatment‐free remission after second‐line nilotinib treatment in patients with chronic myeloid leukemia in chronic phase: results from a single‐group, phase 2, open‐label study. Ann Intern Med. 2018;168(7):461‐470. [DOI] [PubMed] [Google Scholar]
- 32. Giona F, Saglio G, Moleti ML, et al. Treatment‐free remission after imatinib discontinuation is possible in paediatric patients with chronic myeloid leukaemia. Br J Haematol. 2015;168(2):305‐308. [DOI] [PubMed] [Google Scholar]
- 33. Millot F, Claviez A, Leverger G, Corbaciglu S, Groll AH, Suttorp M. Imatinib cessation in children and adolescents with chronic myeloid leukemia in chronic phase. Pediatr Blood Cancer. 2014;61(2):355‐357. [DOI] [PubMed] [Google Scholar]
- 34. Hijiya N, Schultz KR, Metzler M, Millot F, Suttorp M. Pediatric chronic myeloid leukemia is a unique disease that requires a different approach. Blood. 2016;127(4):392‐399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ault P, Kantarjian H, O'Brien S, et al. Pregnancy among patients with chronic myeloid leukemia treated with imatinib. J Clin Oncol. 2006;24(7):1204‐1208. [DOI] [PubMed] [Google Scholar]
- 36. Pye SM, Cortes J, Ault P, et al. The effects of imatinib on pregnancy outcome. Blood. 2008;111(12):5505‐5508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cortes JE, Abruzzese E, Chelysheva E, Guha M, Wallis N, Apperley JF. The impact of dasatinib on pregnancy outcomes. Am J Hematol. 2015;90(12):1111‐1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Abruzzese E, Trawinska MM, Perrotti AP, De Fabritiis P. Tyrosine kinase inhibitors and pregnancy. Mediterr J Hematol Infect Dis. 2014;6(1):e2014028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Shah NP, Wallis N, Farber HW, et al. Clinical features of pulmonary arterial hypertension in patients receiving dasatinib. Am J Hematol. 2015;90(11):1060‐1064. [DOI] [PubMed] [Google Scholar]
- 40. Damrongwatanasuk R, Fradley MG. Cardiovascular complications of targeted therapies for chronic myeloid leukemia. Curr Treat Options Cardiovasc Med. 2017;19(4):24. [DOI] [PubMed] [Google Scholar]
- 41. Lee SE, Choi SY, Song HY, et al. Imatinib withdrawal syndrome and longer duration of imatinib have a close association with a lower molecular relapse after treatment discontinuation: the KID study. Haematologica. 2016;101(6):717‐723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Richter J, Söderlund S, Lübking A, et al. Musculoskeletal pain in patients with chronic myeloid leukemia after discontinuation of imatinib: a tyrosine kinase inhibitor withdrawal syndrome? J Clin Oncol. 2014;32(25):2821‐2823. [DOI] [PubMed] [Google Scholar]
- 43. Shah NP, García Gutiérrez V, Jiménez‐Velasco A, et al. Dasatinib discontinuation in patients (pts) with chronic‐phase chronic myeloid leukemia (CML‐CP) and stable deep molecular response (DASFREE). Blood. 2017;130(Suppl 1):314. [DOI] [PubMed] [Google Scholar]
- 44. Kadowaki N, Kawaguchi T, Kuroda J, et al. Discontinuation of nilotinib in patients with chronic myeloid leukemia who have maintained deep molecular responses for at least 2 years: a multicenter phase 2 Stop Nilotinib (NILSt) trial. Blood. 2016;128:790. [DOI] [PubMed] [Google Scholar]
- 45. Aiello A, Daniel F, D'Ausilio A, Snedecor SJ, Toumi M. Cost‐effectiveness of nilotinib in patients with chronic myeloid leukaemia eligible to start the treatment‐free remission phase in Italy. Value Health. 2015;18:A457. Abstract PCN153. [Google Scholar]
- 46. Goldberg S, Hamarman S. Patients with chronic myelogenous leukemia may not want to discontinue tyrosine kinase inhibitor therapy. Blood. 2015;126:1584. [Google Scholar]
- 47. Riva S, Mazzocco K, Pravettoni G. Rationale and protocol of CML study: cognitive and emotional impact of discontinuation of therapy in patients with chronic myeloid leukemia. Tumori. 2016;102(2):217‐221. [DOI] [PubMed] [Google Scholar]
- 48.CML1113 Front‐line Treatment of BCR‐ABL+ Chronic Myeloid Leukemia (CML) With Dasatinib (CML1113). 2013. ClinicalTrials.gov identifier NCT01761890. https://clinicaltrials.gov/ct2/show/NCT01761890.
- 49. DADI . Dasatinib Discontinuation for Chronic Myelogenous Leukemia‐Chronic Phase with One Year Complete Molecular Remission (DADI). 2011. UMIN‐CTR identifier UMIN000005130.
- 50. IMIDAS . Dasatinib discontinuation for Chronic Myelogenous Leukemia‐Chronic Phase with One Year Complete Molecular Remission (IMIDAS). 2012. UMIN‐CTR identifier UMIN000008999.
- 51. 1st DADI . DAsatinib DIscontinuation for 1st‐Line treatment with Chronic Myelogenous Leukemia‐Chronic Phase with One Year Complete Molecular Remission (1st DADI). 2013. UMIN‐CTR identifier UMIN000011099.
- 52. D‐NewS . Dasatinib for Patients Achieving Complete Molecular Response for Cure D‐NewS Trial. 2013. ClinicalTrials.gov identifier NCT01887561. https://clinicaltrials.gov/ct2/show/NCT01887561.
- 53. STDAST . Clinical research of safety and efficacy after a stop of dasatinib administration in patients with chronic myeloid leukemia ‐chronic phase (CML‐CP) achieving a complete molecular response (CMR) on a treatment of tyrosine kinase inhibitors (TKIs; STDAST). 2012. UMIN‐CTR identifier UMIN000008583.
- 54. Multicenter Clinical Study on the Safety and Efficacy of Dasatinib Discontinuation . Multicenter Clinical Study Evaluating the Safety and Efficacy of Dasatinib Treatment and its Discontinuation in Patients with Chronic Phase Chronic Myelogenous Leukemia who had Reached Complete Molecular Response during Imatinib Therapy. 2012. UMIN‐CTR identifier UMIN000007221.
- 55. D‐FREE . Phase II Clinical Trial of Dasatinib Therapy Aiming for Treatment‐Free Remission in Patients with Chronic Phase Chronic Myeloid Leukemia (D‐FREE). 2016. UMIN‐CTR identifier UMIN000022254.
- 56. Stop Tasigna Trial (STAT2) . Multicenter cooperative clinical study to examine the safety and effectiveness of discontinuation of nilotinib administration for chronic phase chronic myelogenous leukemia patients who have achieved complete molecular response (CMR). 2011. UMIN‐CTR identifier UMIN000005904.
- 57. ENESTPath . A Randomized Phase III Study to Assess the Effect of a Longer Duration of Consolidation Treatment With Nilotinib on TFR in CP CML (ENESTPath). 2012. ClinicalTrials.gov identifier NCT01743989. https://clinicaltrials.gov/ct2/show/NCT01743989.
- 58. TIGER . Tasigna and Interferon Alpha Evaluation Initiated by the German Chronic Myeloid Leukemia Study Group ‐ the TIGER Study. 2012. ClinicalTrials.gov identifier NCT01657604. https://clinicaltrials.gov/ct2/show/NCT01627132NCT01627132.
- 59. LAST . The Life After Stopping Tyrosine Kinase Inhibitors Study (The LAST Study). 2014. ClinicalTrials.gov identifier. NCT02269267. https://clinicaltrials.gov/ct2/show/NCT02269267?term=last+study&rank=2.
- 60. Clark RE, Polydoros F, Apperley JF, et al. De‐escalation of tyrosine kinase inhibitor dose in patients with chronic myeloid leukaemia with stable major molecular response (DESTINY): an interim analysis of a nonrandomised, phase 2 trial. Lancet Haematol. 2017;4(7):e310‐e316. [DOI] [PubMed] [Google Scholar]
- 61. TWMU‐TKI‐STOP‐Study . Stop imatinib or dasatinib study in CP‐CML patients mainitaining complete molecular response for two years (TWMU‐TKI‐STOP‐Study). 2012. UMIN‐CTR identifier UMIN000007944.
- 62. Kumagai T, Nakaseko C, Nishiwaki K, et al. Discontinuation of dasatinib after deep molecular response for over 2 years in patients with chronic myelogenous leukemia and the unique profiles of lymphocyte subsets for successful discontinuation: a prospective, multicenter Japanese trial (D‐STOP trial). Blood. 2016;128:791. [Google Scholar]
- 63. Okada M, Imagawa J, Tanaka H, et al. Final 3‐year results of the dasatinib discontinuation trial in patients with chronic myeloid leukemia who received dasatinib as a second‐line treatment. Clin Lymphoma Myeloma Leuk. 2018;18(5):353‐360.e1. [DOI] [PubMed] [Google Scholar]
- 64. Kim DDH, Bence‐Bruckler I, Forrest DL, et al. Interim results of the Canadian tyrosine kinase inhibitor discontinuation trial for 2nd attempt of treatment free remission: treatment free remission accomplished by Dasatinib (TRAD). Blood. 2017;130(Suppl 11):1622. [Google Scholar]
- 65. Hochhaus A, Masszi T, Giles FJ, et al. Treatment‐free remission following frontline nilotinib in patients with chronic myeloid leukemia in chronic phase: results from the ENESTfreedom study. Leukemia. 2017;31(7):1525‐1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Ross DM, Masszi T, Gómez Casares MT, et al. Durable treatment‐free remission in patients with chronic myeloid leukemia in chronic phase following frontline nilotinib: 96‐week update of the ENESTfreedom study. J Cancer Res Clin Oncol. 2018;144(5):945‐954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Ritchie EK, Catchatourian R, Klisovic RB, et al. ENESTgoal treatment‐free remission study: updated preliminary results and digital polymerase chain reaction analysis in patients with chronic myeloid leukemia in chronic phase who switched from Imatinib to Nilotinib. Blood. 2016;128:3090. [Google Scholar]
- 68. Takahashi N, Nakaseko C, Nishiwaki K, Wakita H. Two‐year consolidation by nilotinib is associated with successful treatment free remission in chronic myeloid leukemia with MR: subgroup analysis from STAT2 trial in Japan. Presented at the 58th Annual Meeting of the American Society of Hematology, December 3‐6, 2016; San Diego, California. Abstract 1889.
- 69. Saussele S, Richter J, Guilhot J, et al. Discontinuation of tyrosine kinase inhibitor therapy in chronic myeloid leukaemia (EURO‐SKI): a prespecified interim analysis of a prospective, multicentre, non‐randomised, trial. Lancet Oncol. 2018;19(6):747‐757. [DOI] [PubMed] [Google Scholar]
- 70. Atallah E, Schiffer CA, Radich JP, et al. Results from the U.S. Life after Stopping TKIs (LAST) study. Blood. 2017;130(Suppl 1):2903. [Google Scholar]
- 71. Thielen N, van der Holt B, Cornelissen JJ, et al. Imatinib discontinuation in chronic phase myeloid leukaemia patients in Sustained complete molecular response: a randomised trial of the Dutch‐Belgian Cooperative Trial for Haemato‐Oncology (HOVON). Eur J Cancer. 2013;49(15):3242‐3246. [DOI] [PubMed] [Google Scholar]
- 72. Rea D, Henry G, Khaznadar Z, et al. Natural killer‐cell counts are associated with molecular relapse‐free survival after imatinib discontinuation in chronic myeloid leukemia: the IMMUNOSTIM study. Haematologica. 2017;102(8):1368‐1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Hughes TP, Boquimpani CM, Benyamini N, et al. Treatment‐free remission in patients with chronic myeloid leukemia in chronic phase according to reasons for switching from imatinib to nilotinib: subgroup analysis from ENESTop. Blood. 2016;128:792. [Google Scholar]
- 74. Fava C, Rege‐Carmbrin G, Dogliotti I, et al. Observational study of CML Italian patients who discontinued TKIs. Blood. 2017;130(Suppl 1):1616. [Google Scholar]
- 75. Tsutsumi Y, Ito S, Ohigashi H, Shiratori S, Teshima T. Unplanned discontinuation of tyrosine kinase inhibitors in chronic myeloid leukemia. Mol Clin Oncol. 2016;4(1):89‐92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Nicolini FE, Alcazer V, Dulucq S, et al. Characterisation of molecular relapse profiles after tyrosine kinase inhibitor cessation in patients eligible for treatment‐free remission strategies in chronic phase CML. Blood. 2016;128:1893. [Google Scholar]
- 77. Hochhaus A, Saglio G, Hughes T, et al. Impact of treatment with frontline nilotinib (NIL) vs imatinib (IM) on sustained deep molecular response (MR) in patients (pts) with newly diagnosed chronic myeloid leukemia in chronic phase (CML‐CP). Blood. 2015;125:2781. [Google Scholar]
- 78. Baccarani M, Deininger MW, Rosti G, et al. European LeukemiaNet recommendations for the management of chronic myeloid leukemia: 2013. Blood. 2013;122(6):872‐884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Arora R, Press RD. Measurement of BCR‐ABL1 transcripts on the International Scale in the United States: current status and best practices. Leuk Lymphoma. 2017;58(1):8‐16. [DOI] [PubMed] [Google Scholar]
- 80. Cross NC, White HE, Colomer D, et al. Laboratory recommendations for scoring deep molecular responses following treatment for chronic myeloid leukemia. Leukemia. 2015;29(5):999‐1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Pfirrmann M, Mahon FX, Guilhot J, et al. No differences in molecular relapse‐free survival after stopping Imatinib treatment of chronic myeloid leukemia between patients with prior 4.5 log reduction (MR4.5) but detectable and patients with undetectable disease in the EURO‐SKI trial. Blood. 2016;128:789. [Google Scholar]
- 82. Dela Cruz JP, Staszewski H. A single center retrospective analysis of adherence to molecular monitoring guidelines in patients with chronic myeloid leukemia. Blood. 2016;128:2365. [Google Scholar]
- 83. Massoud M, Nasr F, Sakr R, Hawi J, Kerbage F, Chahine G. Adherence to monitoring tests in CML patients in Lebanon. Clin Lymphoma Myeloma Leuk. 2016;16(Suppl):S101‐S104. [DOI] [PubMed] [Google Scholar]
- 84. Goh HG, Lin M, Fukushima T, et al. Sensitive quantitation of minimal residual disease in chronic myeloid leukemia using nanofluidic digital polymerase chain reaction assay. Leuk Lymphoma. 2011;52(5):896‐904. [DOI] [PubMed] [Google Scholar]
- 85. Mori S, Vagge E, le Coutre P, et al. The risk of relapse in CML patients who discontinued imatinib can be predicted based on patients age and the results of dPCR analysis. Blood. 2014;124:813. [Google Scholar]
- 86. Legros L, Rousselot P, Giraudier S, et al. Second attempt to discontinue imatinib in CP‐CML patients with a second sustained complete molecular response. Blood. 2012;120(9):1959‐1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Breccia M, Efficace F, Sica S, et al. Adherence and future discontinuation of tyrosine kinase inhibitors in chronic phase chronic myeloid leukemia: a patient‐based survey on 1133 patients. Leuk Res. 2015;39(10):1055‐1059. [DOI] [PubMed] [Google Scholar]


