Abstract
Background
While it is known that CD123 is normally strongly expressed on plasmacytoid dendritic cells and completely absent on nucleated red blood cells, detailed information regarding CD123 expression in acute leukemia is scarce and, if available, hard to compare due to different methodologies.
Methods
CD123 expression was evaluated using standardized EuroFlow immunophenotyping in 139 pediatric AML, 316 adult AML, 193 pediatric BCP‐ALL, 69 adult BCP‐ALL, 101 pediatric T‐ALL, and 28 adult T‐ALL patients. Paired diagnosis‐relapse samples were available for 57 AML and 19 BCP‐ALL patients. Leukemic stem cell (LSC) data was available for 32 pediatric AML patients. CD123 expression was evaluated based on mean fluorescence intensity, median fluorescence intensity, and percentage CD123 positive cells.
Results
EuroFlow panels were stable over time and between laboratories. CD123 was expressed in the majority of AML and BCP‐ALL patients, but absent in most T‐ALL patients. Within AML, CD123 expression was lower in erythroid/megakaryocytic leukemia, higher in NPM1 mutated and FLT3‐ITD mutated leukemia, and comparable between LSC and leukemic blasts. Within BCP‐ALL, CD123 expression was higher in patients with (high) hyperdiploid karyotypes and the BCR‐ABL fusion gene. Interestingly, CD123 expression was increased in BCP‐ALL relapses while highly variable in AML relapses (compared to CD123 expression at diagnosis).
Conclusions
Authors evaluated CD123 expression in a large cohort of acute leukemia patients, based on standardized and reproducible methodology. Our results may facilitate stratification of patients most likely to respond to CD123 targeted therapies and serve as reference for CD123 expression (in health and disease). © 2018 The Authors. Cytometry Part B: Clinical Cytometry published by Wiley Periodicals, Inc. on behalf of International Clinical Cytometry Society.
Keywords: CD123, standardized immunophenotyping, acute leukemia, targeted therapy
The interleukin‐3 receptor alpha chain (IL‐3Rα), more commonly referred to as CD123, is normally strongly expressed on plasmacytoid dendritic cells (PDC) and completely absent on nucleated red blood cells (NRBC). Others have shown that CD123 is widely expressed in various hematological malignancies as well 1, including acute myeloid leukemia (AML) and B cell precursor acute lymphoblastic leukemia (BCP‐ALL). Most notably, it was also shown that CD123 is expressed on both leukemic stem cells (LSC) as well as more differentiated leukemic blasts in both AML and BCP‐ALL, which makes CD123 an attractive therapeutic target 2.
At present, various CD123 therapeutic monoclonal antibodies have entered clinical trials 3. One of these trials is based on the CD123 antibody drug conjugate SGN‐CD123A, where the pyrrolobenzodiazepine toxin was coupled to a CD123 specific antibody 3, leading to targeted drug delivery. Another monoclonal antibody is Talacotuzumab, which was the first CD123 therapeutic monoclonal antibody to enter phase‐III clinical trials. Talacotuzumab is a fully humanized CD123‐neutralizing monoclonal antibody containing a modified Fc structure, which enhances human natural killer cell antibody‐dependent cell‐mediated cytotoxicity, and thereby facilitates lysis of CD123 positive cells.
By targeting a therapeutic monoclonal antibody to the interleukin‐3 receptor alpha chain (CD123), it may block processes regulated by the IL‐3 receptor itself, including cell survival, proliferation, and differentiation 4. Efficacy of CD123 targeted therapies in acute leukemia is likely to correlate with CD123 expression of leukemic cells, as was shown for other targeted therapies. For example, the response to Daratumumab (CD38 targeting therapy) and Gemtuzumab Ozogamicin (CD33 targeting therapy) correlates with CD38 expression and CD33 expression of leukemic cells, respectively 5, 6.
Unfortunately, detailed information regarding CD123 expression in acute leukemia is scarce and, if available, hard to compare between studies due to different methodologies. In this study, we extensively evaluated 846 acute leukemia patients (at diagnosis and at relapse) and 1,736 normal leukocyte populations using highly standardized immunophenotyping protocols and robust statistical analysis. Our data provide a unique insight in CD123 expression (in both health and disease) and may support identification of patients most likely to benefit from novel CD123 targeting therapies.
METHODS
Materials
Acute leukemia patients (n = 846) were retrospectively selected based on availability of relevant flow cytometric data (thus at least one CD123 staining acquired at date of initial diagnosis). Patients were either evaluated by the Dutch Childhood Oncology Group (DCOG) or the Department of Immunology at Erasmus MC (EMC). Included patients were classified according to the WHO 2008 classification 7 and consisted of 139 pediatric AML, 316 adult AML, 193 pediatric BCP‐ALL, 69 adult BCP‐ALL, 101 pediatric T‐ALL and 28 adult T‐ALL patients. Included patients originated from two cohorts of unselected consecutive patients, the pediatric DCOG cohort (initial diagnosis between February 2011 and July 2016) and the pediatric/adult EMC cohort (initial diagnosis between January 2011 and December 2016). Detailed cohort characteristics, including age distributions, are available for the EMC cohort, DCOG cohort and combined EMC‐DCOG (Supporting Information 1). In addition to the initial diagnostic samples, paired relapse samples were available for 57 of the 455 AML patients and 19 of the 262 BCP‐ALL patients. LSC flow cytometry data was available for 32 out of 56 pediatric AML cases from the EMC cohort. Evaluated patients did not receive CD123‐targeted therapy prior to immunophenotyping. In addition to leukemic cell populations, a total of 1736 normal cell populations were identified in 1,252 unique bone marrow (BM) and peripheral blood (PB) samples. Normal cell populations were either identified in samples that were considered normal during diagnostic evaluation and/or samples where the lineage of interest was not affected by disease (e.g., normal lymphocytes in AML and eosinophils in T‐ALL). Besides normal PDC (n = 198) and NRBC (n = 633), which served as negative and positive control populations, also normal eosinophils (n = 67), neutrophils (n = 169), mature B‐lymphocytes (n = 174), T‐lymphocytes (n = 404), and basophils (n = 91) were identified. All samples and resulting datasets were collected according to local Medical Ethics Committee guidelines (METC‐2007‐234).
Immunophenotyping
For each sample, standardized EuroFlow immunophenotyping was performed by either DCOG laboratory or EMC laboratory, both EuroFlow members participating in the EuroFlow Quality Assurance Program 8 and the Dutch Quality Assurance Program 9. Staining and acquisition procedures were performed according to EuroFlow guidelines 10 and flow cytometry was performed on FACSCanto II flow cytometers (BD, Erembodegem, Belgium) with EuroFlow instrument settings 11. Instrument performance was monitored on a daily basis using CS&T beads and rainbow beads 11, 12. BM, PB, lymph node (LN), or pleural effusion (PE) samples were used for immunophenotyping. Samples were processed within 36 h as part of routine diagnostic evaluation and at least 50,000 events were acquired. (Pre‐)analytical procedures were identical for both laboratories. Included AML, BCP‐ALL, and T‐ALL patients were evaluated with the EuroFlow AML/MDS panel, EuroFlow BCP‐ALL panel, and EuroFlow T‐ALL panel, respectively. Each EuroFlow panel contains one eight‐color labeling (Supporting Information 2) with CD123 clone AC145 conjugated with the APC fluorochrome (Miltenyi Biotec, Bergisch Gladbach, Germany), allowing direct comparison of CD123 expression levels. Manual analysis was performed on a single tube basis using Infinicyt software (Cytognos, Salamanca, Spain). Using available markers, the vast majority of malignant populations were clearly distinguishable (Supporting Information 3). Overlapping and doubtful malignant populations (mainly AML cases) were successfully gated by referring to the routine diagnostic evaluation (based on the full EuroFlow panel). Gating strategies were different for each hematological malignancy, but never included any CD123 gates. Normal populations were only included in cases where they were clearly distinguishable and presented with a normal immunophenotype (Supporting Information 4). Detailed information regarding the LSC assay was previously published and a similar gating strategy was used (Supporting Information 5).
Assay Stability
Assay stability was evaluated in 633 normal NRBC populations (as negative reference) and 198 normal PDC populations (as positive reference). To evaluate assay stability over time, between laboratories and across panels, the normal NRBC and PDC were grouped by acquisition year, acquisition laboratory, and/or acquisition panel. Subsequently, the groups were evaluated for differences in variance and distribution.
Statistical Analysis
CD123 median fluorescence intensity (CD123‐MFI) and percentage CD123 positive cells (CD123‐PPC) were used as measurement for CD123 expression. Cut‐off for CD123 positivity was defined as the highest 98th percentile found among all 633 normal NRBC populations. Impact of the chosen CD123 positivity cut‐off was evaluated by repeating statistical analysis based on various (arbitrarily chosen) lower and higher positivity cut‐offs. Impact of using median instead of mean fluorescence intensity was evaluated as well. Mann–Whitney U tests (two tailed significance, paired where appropriate) were used to compare the means of two subgroups. In case of numerous comparisons, means of subgroups were compared using one‐way analysis of variance (ANOVA). Brown–Forsythe test, Fligner‐Killeen test, and Barlet test were used to evaluate homogeneity of variances of multiple subgroups. Pearson correlation coefficient was used as measurement for linear correlation and Spearman's rank correlation coefficient was used as measure for rank correlation.
Relationship between two variables was considered significant when both Pearson's and Spearman's coefficient were significant. Interpretation of results was performed at an alpha level of 0.05 after applying Bonferroni multiple testing corrections where appropriate. Statistical analysis was performed in R base (version 3.4.1) with the Car library for variance testing (version 2.1.4) and the FlowCore library for extraction, compensation, and transformation of manually gated FCS files (version 1.42.2).
RESULTS
Assay Stability
Normal NRBC and PDC populations were identified in samples acquired at different time points (2011–2016), in different laboratories (DCOG or EMC) and using different panels (EuroFlow AML/MDS, BCP‐ALL, and T‐ALL). Resulting populations were grouped by year and laboratory to evaluate assay stability over time and across laboratories (Fig. 1). Overall comparison of the subgroups (using ANOVA, Brown–Forsythe, Fligner‐Killeen, and Barlet) revealed no significant differences (always P > 0.05). Comparison of individual subgroups versus the remainder of subgroups together (using Mann–Whitney U with Bonferroni correction) revealed no significant differences as well. Therefore, there was no statistical evidence to suggest that the NRBC subgroups or PDC subgroups have different distributions and/or variances. Grouping the populations by year and by panel showed no significant differences as well (Supporting Information 6). Overall, these data indicated that the applied CD123 assay was stable and reproducible for the duration of the study (over time and across laboratories).
Figure 1.
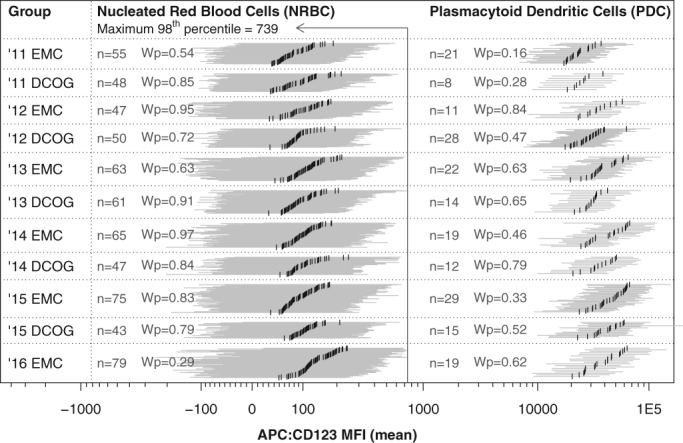
Assay stability over time (2011–2016) and across laboratories (EMC and DCOG). Evaluation based on normal NRBC and normal PDC (as negative and positive control, respectively). Visualization per individual population, where the horizontal gray bars visualize the 96% interval and the vertical black bars visualize the median CD123 expression (CD123‐MFI). Solid vertical line indicates the highest 98 percentile found among all NRBC populations, which was used as positivity cut‐off during CD123‐PPC analysis. For each subgroup, the Wilcoxon signed‐rank test result is shown, comparing the subgroup against the remainder of populations (Wp denotes the probability value after Bonferroni correction). One‐way ANOVA did not reveal any significant differences among the NRBC and PDC subgroups as well (both tests P > 0.05). The Bartlett test, Fligner‐Killeen test, and Brown–Forsythe tests, comparing all PDC subgroups at once, did not reveal any significant differences as well (P = 0.154, P = 0.065, P = 0.075, respectively). In conclusion, evaluated subgroups have comparable distributions and variances, confirming assay stability over time and across centers.
Positivity Cut‐Off
Evaluated normal NRBC populations were used to determine the cut‐off for CD123 positivity. CD123 positivity cut‐off was defined as the highest 98th percentile found among all normal NRBC populations. Based on this positivity cut‐off, located at 739 intensity (Fig. 1), only low numbers of NRBC were identified to be CD123 positive (less than 2% positive cells within all NRBC populations and even less than 1% positive cells within 95% of NRBC populations). Various arbitrary intensity cut‐offs (700, 800, 900, and 1000) were evaluated as well (Supporting Information 7). For each arbitrary cut‐off, the highest percentage CD123 positive cells (found among all NRBC populations) was still low (2.4, 1.7, 1.6, and 1.5%, respectively). During this study, CD123 positivity was evaluated based on the 739 intensity cut‐off (as it was objectively defined) and evaluated based on the 1,000 intensity cut‐off (to make sure our results were not influenced by CD123 false‐positive events).
CD123‐MFI in Normal Cells
Average CD123 expression levels, as measured by CD123 median fluorescence intensity (CD123‐MFI), were visualized for each normal and malignant population on a default logical scale (Fig. 2A). As expected, compared to any other normal and abnormal population, CD123‐MFI was lowest in NRBC (P < 0.001) and highest in PDC (P < 0.001). Other evaluated normal populations showed significant different CD123‐MFI levels as compared to each other (P < 0.001), where CD123‐MFI was lowest in T‐cells and increasingly higher in mature B‐cells, granulocytes, eosinophils, and basophils. Within all evaluated normal populations CD123 expression was homogeneous. CD123 expression was also evaluated in a limited set of normal myeloid and B‐cell precursors. CD123‐MFI was low in CD34‐positive BCP, higher in CD34‐negative BCP (P < 0.001) and highest in CD34‐positive myeloid precursors (P < 0.001; Supporting Information 8), in line with literature 13.
Figure 2.
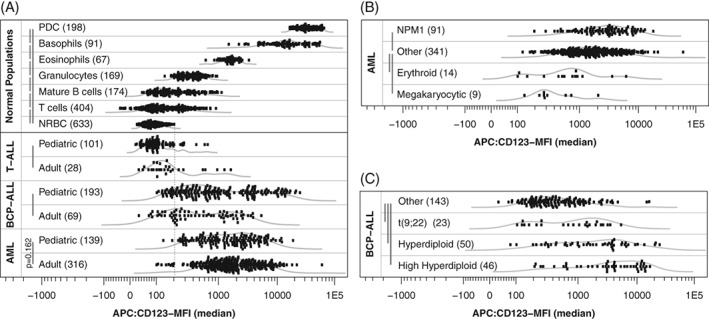
CD123 median fluorescence intensity (CD123‐MFI) for normal mature leukocyte subsets and acute leukemia. Curved gray lines visualize Gaussian kernel density estimation. Vertical gray bars visualize performed Wilcoxon signed‐rank tests (P‐value after Bonferroni multiple testing correction was smaller than 0.001 unless otherwise specified). (A) CD123‐MFI for AML, BCP‐ALL, and T‐ALL patients and various normal populations. Vertical dotted line represents the highest CD123‐MFI among evaluated NRBC populations. (B) CD123‐MFI levels for selected AML subgroups. (C) CD123‐MFI levels for selected BCP‐ALL subgroups.
CD123‐MFI in Malignant Cells
CD123‐MFI was low in T‐ALL although significantly higher compared to NRBC (Fig. 2A). In contrast, CD123‐MFI was high in AML and BCP‐ALL compared to NRBC (Fig. 2A). Only 2% of AML cases (11/455) and 15% of BCP‐ALL cases (38/262) showed lower CD123‐MFI than the highest CD123‐MFI observed among all normal NRBC populations (dotted vertical line).
Pediatric BCP‐ALL cases had significantly higher CD123‐MFI while pediatric T‐ALL cases had significantly lower CD123‐MFI compared to their adult counterparts (Fig. 2A). T‐ALL patients with notable high CD123‐MFI did not have any obvious characteristics in common (including immunophenotypic maturation stage and/or being early T‐cell precursor T‐ALL). No significant difference in CD123‐MFI was present between pediatric and adult AML (Fig. 2A). In addition, continuous analysis (Supporting Information 9) revealed that age negatively correlated to CD123‐MFI in BCP‐ALL, positively correlated to CD123‐MFI in T‐ALL and did not correlate to CD123‐MFI in AML. Although significant these age correlations are weak and likely clinical irrelevant.
Relation between CD123‐MFI and WHO 2008 classification was evaluated for BCP‐ALL and AML patients. Within AML, CD123‐MFI was significantly lower in patients with the erythroid or megakaryocytic lineage affected and significantly higher in patients with a mutated NPM1 (Fig. 2B). FLT3‐ITD positive AML patients had significant (P < 0.001) higher CD123‐MFI as compared to FLT3‐ITD negative AML patients (Supporting Information 9). Increased CD123‐MFI expression in NPM1 patients was not caused by co‐existing FLT3‐ITD (as the majority of these patients were FLT3‐ITD negative). Within BCP‐ALL CD123‐MFI was significantly higher in (high) hyperdiploid patients and patients with the BCR‐ABL1 fusion gene (Fig. 2C). Other genetic characteristics did not relate to CD123‐MFI in AML and BCP‐ALL (Supporting Information 10).
CD123‐PPC in Malignant Cells
Next the percentage of CD123‐positive cells based on the 739 positivity cut‐off (CD123‐PPC) was evaluated for AML patients, BCP‐ALL patients, and T‐ALL patients (Fig. 3A). CD123‐PPC was highest in AML, lower in BCP‐ALL, and lowest in T‐ALL (all comparisons P < 0.001). At least 80% of leukemic cells were CD123 positive in approximately 55% of AML patients and 30% of BCP‐ALL patients. Pediatric BCP‐ALL patients showed significantly higher CD123‐PPC while pediatric T‐ALL cases showed significantly lower CD123‐PPC compared to their adult counterparts (P < 0.01). No significant difference in CD123‐PPC was observed between pediatric and adult AML. Continuous analysis (Supporting Information 11) revealed that age was negatively correlated to CD123‐PPC in BCP‐ALL and positively correlated to CD123‐PPC in T‐ALL. Relation between CD123‐PPC and WHO 2008 classification was evaluated for BCP‐ALL and AML patients. Within AML, the CD123‐PPC was significantly lower in patients with the erythroid or megakaryocytic lineage affected and significant higher in patients with a mutated NPM1 (Fig. 3B). Within BCP‐ALL, the CD123‐PPC was significantly higher in (high) hyperdiploid patients and patients with the BCR‐ABL1 fusion gene (Fig. 3C). CD123‐PPC was significantly higher in FLT3‐ITD positive AML patients (P < 0.001). Other genetic characteristics did not relate to CD123‐PPC in AML and BCP‐ALL (Supporting Information 12). In conclusion, results based on CD123‐PPC were comparable to the results based on CD123‐MFI.
Figure 3.
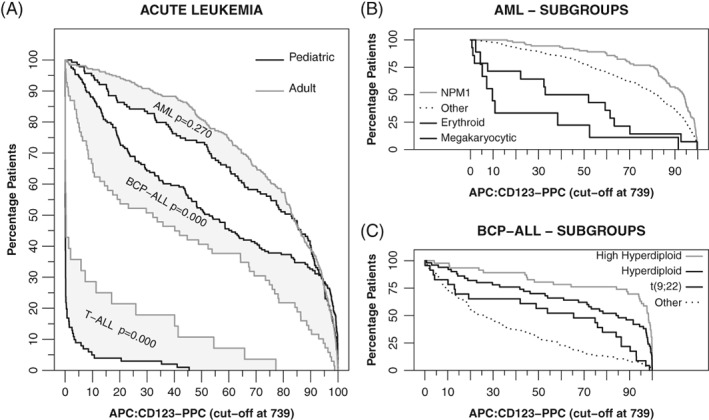
Percentage CD123 positive cells (CD123‐PPC) for AML, BCP‐ALL, and T‐ALL patients. Percentage CD123 positive cells based on 739 intensity cut‐off. Black lines visualize pediatric patients and gray lines visualize adult patients. Gray area visualizes the difference between pediatric and adult patients (reported P‐values based on a Mann–Whitney U tests). Other immunophenotyping studies typically state “at least … patients have at least … percent positive cells.” As different studies use different percentages, we have chosen for this visualization, which allows lookup of any combination. For example, approximately 65% of pediatric AML patients have at least 60% CD123 positive cells based on the 739 positivity cut‐off. (A) CD123‐PPC for AML, BCP‐ALL and T‐ALL patients. (B) CD123‐PPC for selected AML subgroups. (C) CD123‐PPC for selected BCP‐ALL subgroups.
CD123‐MFI in Relapses and LSC
Paired diagnosis‐relapse samples were evaluated for 19 BCP‐ALL patients and 57 AML patients. Within BCP‐ALL, the overall CD123‐MFI levels were significantly higher at relapse as compared to diagnosis, with only 3 out of 19 patients showing slightly lower CD123 expression at relapse (Fig. 4A). Within AML, the overall CD123‐MFI levels (diagnosis vs. relapse) were not significantly different (Fig. 4B). However, within AML the CD123‐MFI levels on a per patient basis were highly variable between diagnosis and relapse, showing either significant gains or losses. Observed CD123 gains and losses did not appear to be caused by CD123 negative and/or positive subclones as the vast majority featured unimodal CD123 distributions (Supporting Information 13). Gains and losses did not correlate to WHO classifications and/or time‐till‐relapse (Supporting Information 14).
Figure 4.
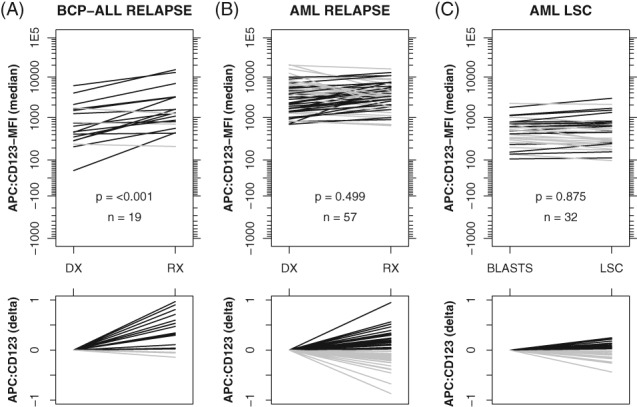
Paired evaluations (diagnosis versus relapse and leukemic blasts vs. leukemic stem cells). Initial diagnosis (DX) versus relapse (RX) for BCP‐ALL patients (A) and AML patients (B). Leukemic blasts versus LSCs in AML patients (C). Black bars indicate increased CD123‐MFI while gray bars indicate decreased CD123‐MFI (right vs. left side). Lower panels visualize the delta CD123 expression (after default logicle transformation), thereby clearly visualizing the magnitude and direction of changes. Reported P‐values are based on a paired Mann–Whitney U test.
Within AML, the CD123‐MFI of LSC (defined as the CD34 positive and CD38 negative fraction of leukemic blasts) was highly comparable to CD123‐MFI of non‐LSC (defined as the remainder of the leukemic blasts). The vast majority of LSC and non‐LSC populations featured a unimodal CD123 distribution (Supporting Information 15).
Robustness
CD123 expression was evaluated based on two distinct measurements; average CD123 expression (as measured by CD123 median fluorescence intensity, referred to as CD123‐MFI) and the number of CD123 positive cells (as measured by percentage CD123 positive cells based on 739 intensity cut‐off, referred to as CD123‐PPC). Statistical analysis based on CD123‐MFI and CD123‐PPC resulted in identical conclusions.
Average expression is typically measured by either mean or median fluorescence intensity, which potentially influences results. Although expression patterns generally seemed to be normally distributed, statistical tests based on CD123 median fluorescence intensity values were repeated based on CD123 mean fluorescence intensity values; this resulted in identical conclusions (Ext Supporting Information 1).
Number of positive cells can be measured based on any intensity cut‐off, whereby lower cut‐offs might introduce more false‐positive cells. Therefore, statistical tests based on the objectively defined 739 positivity cut‐off were also repeated based on the arbitrarily chosen 1,000 intensity cut‐off, which resulted in identical conclusions as well, thereby ruling out a (strong) influence of false‐positive cells on the final conclusions (Ext Supporting Information 2).
Within the combined EMC‐DCOG cohort, the age distribution of AML and T‐ALL patients was skewed toward pediatric cases (Supporting Information 1), therefore each analysis including age as statistical parameter was repeated within EMC cohort (consisting of consecutive pediatric and adult cases), which resulted in identical conclusions (Ext Supporting Information 3 for CD123‐MFI correlations and Ext Supporting Information 4 for CD123‐PPC correlations).
CONCLUSION AND DISCUSSION
Routine diagnostic evaluation based on highly standardized immunophenotyping techniques allowed us to retrospectively evaluate CD123 expression in 846 acute leukemia patients and 1,736 normal populations. To our best knowledge, this is the first time that samples from so many patients were analyzed in a fully standardized manner, allowing quantification of CD123 expression in acute leukemia.
Extensive validation confirmed assay stability over time and across laboratories, therefore these results (including absolute fluorescent intensities) should be fully reproducible by laboratories using EuroFlow immunophenotyping protocols and EuroFlow instrument settings. General conclusions (including relative fluorescent intensities) should be fully reproducible by any laboratory (also using different protocols and/or fluorochromes).
Observed minor nonsignificant changes over time and across laboratories (Fig. 1) most likely resulted from lot‐to‐lot variations and/or technical variation after cytometer maintenance. Evaluated positive controls (PDC) feature very strong CD123 expression, therefore, small changes in expression might be exaggerated by the nonlinear nature of PMT at high intensities. The vast majority of evaluated malignancies showed CD123 expression well within the linear region of the PMT, therefore, it seems unlikely that these nonsignificant changes influenced the overall results.
Our conclusions were based on robust statistical analysis, whereby various common analysis strategies resulted in similar conclusions. Conclusions were not impacted by the chosen measurement (CD123‐MFI vs. CD123‐PPC), chosen descriptive statistic (median vs. mean), chosen CD123 positivity cut‐off (739 vs. 1000) nor chosen cohort (EMC cohort vs. EMC‐DCOG cohort).
In general, CD123 was expressed in the vast majority of AML and BCP‐ALL patients, while absent in most T‐ALL patients. Others have indeed shown that CD123 is only expressed in the minority of T‐ALL patients 14. Within AML patients, CD123 expression was higher in NPM1 and FLT1‐TD mutated patients and lower in cases in whom the erythroid or megakaryocytic lineage was affected, confirming previous smaller studies 2, 15, 16. FLT3‐mutated AML are poor risk patients that therefore could be prime candidates for CD123‐targeting strategies. Within BCP‐ALL patients, CD123 expression was higher in patients with a (high) hyperdiploid karyotype or the BCR‐ABL1 fusion gene, confirming previous reports as well 2, 17.
Interestingly, paired BCP‐ALL diagnosis‐relapse samples revealed that CD123 expression at relapse was considerably higher compared to initial diagnosis, indicating CD123 targeted therapy as an interesting treatment option in relapsed BCP‐ALL. Based on available flow cytometric data, no relation with maturation status was found, therefore the reason for this higher CD123 expression in relapsed BCP‐ALL remains unclear. In contrast, CD123 expression in paired AML diagnosis‐relapse samples was highly variable, showing either considerably higher or lower CD123 expression. Variable immunophenotype changes between diagnosis and relapse in AML have been reported for other markers as well 18, 19.
Flow cytometry‐based minimal‐residual disease (MRD) analysis in BCP‐ALL is mainly limited due to lack of leukemia associated immunophenotypes (LAIPs) and drug‐induced antigen modulation 20. CD123 has a strong rationale toward flow cytometry‐based MRD analysis 20 and contributes significantly to newly identified LAIPs 20, 21, 22, 23.
Interestingly, we observed that the expression of CD123 was highly comparable for LSC and the remainder of leukemic blasts, which is especially of interest as LSC within AML patients have an inherent resistance to traditional chemotherapeutics and their persistence following chemotherapy is often considered to be responsible for disease relapse 24, 25. In addition, others have shown that CD123 is expressed on a majority of AML leukemic stem cells while only on a minority of the normal hematopoietic stem cells 26, advocating an interesting therapeutic target for AML treatment. Taken together, CD123 targeted therapy might be of high value for CD123 positive AML cases, both at diagnosis and at relapse.
One concern for CD123 targeted therapy might be the relatively high CD123 expression in various normal populations (eosinophils, neutrophils, plasmacytoid dendritic cells), which might lead to eradication of these cells during therapy. It remains to be seen in clinical trials how critical this will be with respect to toxicity and safety for patients. Although first studies showed that CD123 therapy is generally well tolerated 27, a recent clinical trial evaluating the efficacy of Talacotuzumab has been put on hold for yet unknown reasons.
Several CD123 therapeutic monoclonal antibodies have recently been developed and their efficacy is likely to correlate with CD123 expression levels. However, it should be emphasized that the expression levels do not necessarily translate in CD123 antibody therapy efficacy, as therapy efficacy depends on many factors as well, including antigen density, antigen distribution, antigen affinity, internalization rate, association rate, and dissociation rate 28, 29, 30.
Given the recent number of publications regarding CD123 therapeutic agents and the recent initiation of various clinical trials, insight in CD123 expression is in high demand. Altogether, our results provide a trustworthy and unique insight into CD123 expression. Results from previous smaller sized studies were confirmed in a large cohort of patients using standardized and reproducible methodology. Increased expression in relapsing BCP‐ALL is a novel finding (and potentially very interesting for future therapy decisions and MRD panel design). Our data may facilitate stratification of patients most likely to respond to CD123 targeted therapies and may serve as reference for CD123 expression in both health and disease.
AUTHORSHIP
V.H.J.v.d.V. and A.E.B. designed the study; V.d.H., M.J.L., H.B.B., and M.C.Z. provided patient material and clinical data; J.G.t.M. performed laboratory research; A.E.B. and V.H.J.v.d.V. analyzed data. A.E.B., J.J.M.v.D. and V.H.J.v.d.V. interpreted initial results; A.E.B., V.H.J.v.d.V., A.S., and J.H.W.L. wrote the manuscript; J.J.M.v.D. and V.H.J.v.d.V. were responsible for financial support; finally all authors critically reviewed the manuscript and gave their approval.
CONFLICTS OF INTEREST
None were disclosed by the authors.
Supporting information
Appendix S1: Supporting Information
Appendix S2: Supporting Information
Appendix S3: Supporting Information
Appendix S4: Supporting Information
ACKNOWLEDGMENTS
We gratefully acknowledge all clinicians participating in this study for providing patient material and clinical data. We gratefully thank all technicians of the Laboratory Medical immunology for their support. The research for this manuscript was performed within the framework of the Erasmus Postgraduate School Molecular Medicine.
How to cite this article: Bras AE, de Haas V, van Stigt A, Jongen‐Lavrencic M, Beverloo HB, te Marvelde JG, Zwaan CM, van Dongen JJM, Leusen JHW, and van der Velden VHJ. CD123 expression levels in 846 acute leukemia patients based on standardized immunophenotyping. Cytometry Part B 2019; 96B: 134–142.
LITERATURE CITED
- 1. Munoz L, Nomdedeu JF, Lopez O, Carnicer MJ, Bellido M, Aventin A, Brunet S, Sierra J. Interleukin‐3 receptor alpha chain (CD123) is widely expressed in hematologic malignancies. Haematologica. 2001;86:1261–1269. [PubMed] [Google Scholar]
- 2. Testa U, Pelosi E, Frankel A. CD 123 is a membrane biomarker and a therapeutic target in hematologic malignancies. Biomark Res. 2014;2:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Buckley SA, Walter RB. Update on antigen‐specific immunotherapy of acute myeloid leukemia. Curr Hematol Malig Rep. 2015;10:65–75. [DOI] [PubMed] [Google Scholar]
- 4. Garfin PM, Feldman EJ. Antibody‐based treatment of acute myeloid leukemia. Curr Hematol Malig Rep. 2016;11:545–552. [DOI] [PubMed] [Google Scholar]
- 5. Nijhof IS, Casneuf T, van Velzen J, van Kessel B, Axel AE, Syed K, Groen RW, van Duin M, Sonneveld P, Minnema MC, et al. CD38 expression and complement inhibitors affect response and resistance to daratumumab therapy in myeloma. Blood. 2016;128:959–970. [DOI] [PubMed] [Google Scholar]
- 6. Walter RB, Gooley TA, van der Velden VH, Loken MR, van Dongen JJ, Flowers DA, Bernstein ID, Appelbaum FR. CD33 expression and P‐glycoprotein‐mediated drug efflux inversely correlate and predict clinical outcome in patients with acute myeloid leukemia treated with gemtuzumab ozogamicin monotherapy. Blood. 2007;109:4168–4170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Campo E, Swerdlow SH, Harris NL, Pileri S, Stein H, Jaffe ES. The 2008 WHO classification of lymphoid neoplasms and beyond: Evolving concepts and practical applications. Blood. 2011;117:5019–5032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kalina T, Flores‐Montero J, Lecrevisse Q, Pedreira CE, van der Velden VH, Novakova M, Mejstrikova E, Hrusak O, Bottcher S, Karsch D, et al. Quality assessment program for EuroFlow protocols: Summary results of four‐year (2010‐2013) quality assurance rounds. Cytometry A. 2015;87A:145–156. [DOI] [PubMed] [Google Scholar]
- 9. Preijers FW, van der Velden VH, Preijers T, Brooimans RA, Marijt E, Homburg C, van Montfort K, Gratama JW, Section I. Molecular cell diagnostics of the Foundation for Quality Assessment in Medical L. Fifteen years of external quality assessment in leukemia/lymphoma immunophenotyping in the Netherlands and Belgium: A way forward. Cytometry B Clin Cytom. 2016;90B:267–278. [DOI] [PubMed] [Google Scholar]
- 10. van Dongen JJ, Lhermitte L, Bottcher S, Almeida J, van der Velden VH, Flores‐Montero J, Rawstron A, Asnafi V, Lecrevisse Q, Lucio P, et al. EuroFlow antibody panels for standardized n‐dimensional flow cytometric immunophenotyping of normal, reactive and malignant leukocytes. Leukemia. 2012;26:1908–1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kalina T, Flores‐Montero J, van der Velden VH, Martin‐Ayuso M, Bottcher S, Ritgen M, Almeida J, Lhermitte L, Asnafi V, Mendonca A, et al. EuroFlow standardization of flow cytometer instrument settings and immunophenotyping protocols. Leukemia. 2012;26:1986–2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mizrahi O, Ish Shalom E, Baniyash M, Klieger Y. Quantitative flow cytometry: Concerns and recommendations in clinic and research. Cytometry B Clin Cytom. 2018;94B:211–218. [DOI] [PubMed] [Google Scholar]
- 13. Hassanein NM, Alcancia F, Perkinson KR, Buckley PJ, Lagoo AS. Distinct expression patterns of CD123 and CD34 on normal bone marrow B‐cell precursors ("hematogones") and B lymphoblastic leukemia blasts. Am J Clin Pathol. 2009;132:573–580. [DOI] [PubMed] [Google Scholar]
- 14. Du W, Li J, Liu W, He Y, Yao J, Liu Y, Lin J, Zheng J. Interleukin‐3 receptor alpha chain (CD123) is preferentially expressed in immature T‐ALL and may not associate with outcomes of chemotherapy. Tumour Biol. 2016;37:3817–3821. [DOI] [PubMed] [Google Scholar]
- 15. Ehninger A, Kramer M, Rollig C, Thiede C, Bornhauser M, von Bonin M, Wermke M, Feldmann A, Bachmann M, Ehninger G, et al. Distribution and levels of cell surface expression of CD33 and CD123 in acute myeloid leukemia. Blood Cancer J. 2014;4:e218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chavez‐Gonzalez A, Dorantes‐Acosta E, Moreno‐Lorenzana D, Alvarado‐Moreno A, Arriaga‐Pizano L, Mayani H. Expression of CD90, CD96, CD117, and CD123 on different hematopoietic cell populations from pediatric patients with acute myeloid leukemia. Arch Med Res. 2014;45:343–350. [DOI] [PubMed] [Google Scholar]
- 17. Djokic M, Bjorklund E, Blennow E, Mazur J, Soderhall S, Porwit A. Overexpression of CD123 correlates with the hyperdiploid genotype in acute lymphoblastic leukemia. Haematologica. 2009;94:1016–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. van der Velden VH, van der Sluijs‐Geling A, Gibson BE, te Marvelde JG, Hoogeveen PG, Hop WC, Wheatley K, Bierings MB, Schuurhuis GJ, de Graaf SS, et al. Clinical significance of flowcytometric minimal residual disease detection in pediatric acute myeloid leukemia patients treated according to the DCOG ANLL97/MRC AML12 protocol. Leukemia. 2010;24:1599–1606. [DOI] [PubMed] [Google Scholar]
- 19. Zeijlemaker W, Gratama JW, Schuurhuis GJ. Tumor heterogeneity makes AML a "moving target" for detection of residual disease. Cytometry B Clin Cytom. 2014;86B:3–14. [DOI] [PubMed] [Google Scholar]
- 20. Tembhare PR, Ghogale S, Ghatwai N, Badrinath Y, Kunder N, Patkar NV, Bibi AR, Chatterjee G, Arora B, Narula G, et al. Evaluation of new markers for minimal residual disease monitoring in B‐cell precursor acute lymphoblastic leukemia: CD73 and CD86 are the most relevant new markers to increase the efficacy of MRD 2016; 00B: 000‐000. Cytometry B Clin Cytom. 2018;94B:100–111. [DOI] [PubMed] [Google Scholar]
- 21. Dworzak MN, Buldini B, Gaipa G, Ratei R, Hrusak O, Luria D, Rosenthal E, Bourquin JP, Sartor M, Schumich A, et al. AIEOP‐BFM consensus guidelines 2016 for flow cytometric immunophenotyping of pediatric acute lymphoblastic leukemia. Cytometry B Clin Cytom. 2018;94B:82–93. [DOI] [PubMed] [Google Scholar]
- 22. Theunissen P, Mejstrikova E, Sedek L, van der Sluijs‐Gelling AJ, Gaipa G, Bartels M, Sobral da Costa E, Kotrova M, Novakova M, Sonneveld E, et al. Standardized flow cytometry for highly sensitive MRD measurements in B‐cell acute lymphoblastic leukemia. Blood. 2017;129:347–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sedek L, Theunissen P, Sobral da Costa E, van der Sluijs‐Gelling A, Mejstrikova E, Gaipa G, Sonsala A, Twardoch M, Oliveira E, Novakova M, et al. Differential expression of CD73, CD86 and CD304 in normal vs. leukemic B‐cell precursors and their utility as stable minimal residual disease markers in childhood B‐cell precursor acute lymphoblastic leukemia. J Immunol Methods. 2018. 10.1016/j.jim.2018.03.005 [DOI] [PubMed] [Google Scholar]
- 24. Xie LH, Biondo M, Busfield SJ, Arruda A, Yang X, Vairo G, Minden MD. CD123 target validation and preclinical evaluation of ADCC activity of anti‐CD123 antibody CSL362 in combination with NKs from AML patients in remission. Blood Cancer J. 2017;7:e567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jin L, Lee EM, Ramshaw HS, Busfield SJ, Peoppl AG, Wilkinson L, Guthridge MA, Thomas D, Barry EF, Boyd A, et al. Monoclonal antibody‐mediated targeting of CD123, IL‐3 receptor alpha chain, eliminates human acute myeloid leukemic stem cells. Cell Stem Cell. 2009;5:31–42. [DOI] [PubMed] [Google Scholar]
- 26. Pelosi E, Castelli G, Testa U. Targeting LSCs through membrane antigens selectively or preferentially expressed on these cells. Blood Cells Mol Dis. 2015;55:336–346. [DOI] [PubMed] [Google Scholar]
- 27. He SZ, Busfield S, Ritchie DS, Hertzberg MS, Durrant S, Lewis ID, Marlton P, McLachlan AJ, Kerridge I, Bradstock KF, et al. A phase 1 study of the safety, pharmacokinetics and anti‐leukemic activity of the anti‐CD123 monoclonal antibody CSL360 in relapsed, refractory or high‐risk acute myeloid leukemia. Leuk Lymphoma. 2015;56:1406–1415. [DOI] [PubMed] [Google Scholar]
- 28. van der Velden VH, Boeckx N, Jedema I, te Marvelde JG, Hoogeveen PG, Boogaerts M, van Dongen JJ. High CD33‐antigen loads in peripheral blood limit the efficacy of gemtuzumab ozogamicin (Mylotarg) treatment in acute myeloid leukemia patients. Leukemia. 2004;18:983–988. [DOI] [PubMed] [Google Scholar]
- 29. de Vries JF, Zwaan CM, De Bie M, Voerman JS, den Boer ML, van Dongen JJ, van der Velden VH. The novel calicheamicin‐conjugated CD22 antibody inotuzumab ozogamicin (CMC‐544) effectively kills primary pediatric acute lymphoblastic leukemia cells. Leukemia. 2012;26:255–264. [DOI] [PubMed] [Google Scholar]
- 30. Rudnick SI, Adams GP. Affinity and avidity in antibody‐based tumor targeting. Cancer Biother Radiopharm. 2009;24:155–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1: Supporting Information
Appendix S2: Supporting Information
Appendix S3: Supporting Information
Appendix S4: Supporting Information


