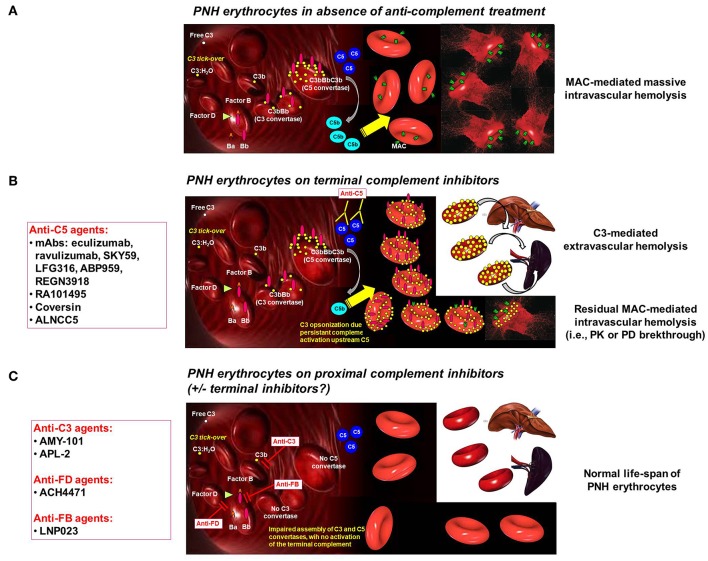
Figure 1.
Complement activation on PNH erythrocytes. (A) PNH erythrocytes inabsence of anti-complement treatment. The complement system may activate due to different triggers through the alternative, classical and mannose/lectin pathway. Spontaneous C3 tick-over continuously generates low-grade activation of the alternative pathway in the fluid phase and possible binding of activated C3 fragments on erythrocytes. Due to the lack of CD55, this leads on PNH erythrocytes to the generation of C3 convertase, with further generation of C3b, which eventually leads to the assembly of C5 convertase. Then, the terminal pathway of the complement cascade is activated, with the generation of the MAC, eventually leading to lysis of PNH erythrocytes lacking CD59. (B) PNH erythrocytes on terminal complement inhibitors. Terminal complement inhibitors (i.e., anti-C5 agents) prevent the cleavage of C5 into C5a and C5b, thereby disabling the formation of the MAC. Thus, PNH erythrocytes are largely protected from intravascular lysis. Nevertheless, early phases of surface complement activation remain uncontrolled on PNH erythrocytes due to the lack of CD55; thus, continuous low-grade activation continues leads to opsonization of PNH erythrocytes with C3 fragments. This excess of C3 generates high-affinity C5 convertases, which may account for residual intravascular hemolysis due to pharmacodynamic breakthrough (in addition to possible pharmacokinetic breakthrough due to sub-therapeutic plasma leven of anti-C5 agent). Moreover, C3 opsonization leads to extravascular hemolysis due to C3-specific receptors expressed on professional macrophages in the liver and in the spleen. (C) PNH erythrocytes on proximal complement inhibitors(± terminal complement inhibitors). Proximal complement inhibitors intercept complement activation at the level of its key component C3 (i.e., anti-C3 agents), or even upstream at the level of initial activation of the alternative pathway (i.e., anti-FD and anti-FB agents). All these agents prevent early activation of complement on the surface of PNH erythrocytes, counterbalancing the deficiency of the complement regulators CD55 and CD59. Based on theoretical assumptions and in vitro data, proximal complement inhibitors prevent C3 opsonization, thereby preventing C3-mediated extravascular hemolysis. However, by disabling early surface complement activation, proximal complement inhibitors should also prevent intravascular hemolysis. While preliminary clinical data already confirmed that proximal complement inhibitors prevent C3-mediated extravascular hemolysis, ongoing investigation will make clear whether they can adequately prevent intravascular hemolysis even in the absence of terminal inhibitors (as already documented in vitro).