Figure 2.
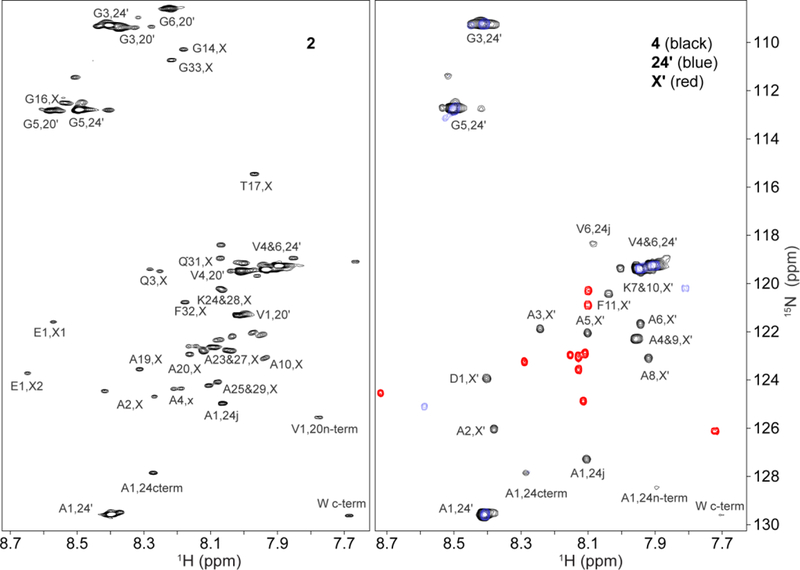
NMR spectral assignments of designed elastin proteins 2 and 4 and their component domains. 15N HSQC peaks from the isolated hydrophobic module, 24’ (blue), and cross-linker, X’ (red), are superimposed on spectra (black peaks) of minielastins 2 (left) and 4 (right) that contain them. Assignments indicate the module and the residue position within the module. For example, A1,24 is the first residue in the APGVGV repeat of the 24’ hydrophobic module and A4,X or A4,X’ are the fourth residues in the 2123 or X’ cross-link modules, respectively.
