Figure 5.
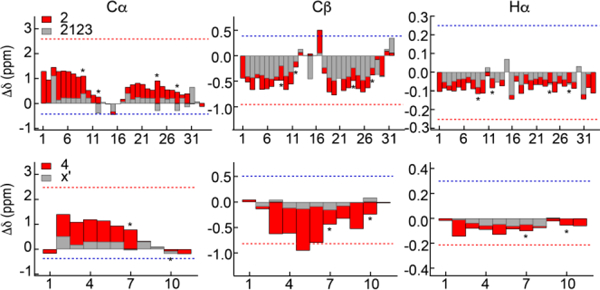
Helical structure in elastin crosslinking domains. Secondary shifts for the cross-link modules, 2123 and X’, with (2 and 4, red bars) and without (2123 and X’, gray bars) flanking 24’ hydrophobic modules. Gray bars are superimposed on red bars and asterisks indicate positions of lysine residues. Numbers refer to sequences in Table 1. Dotted lines indicate an average of the calculated secondary shifts for the sequences constrained to helix (red) and extended (blue) structures.
