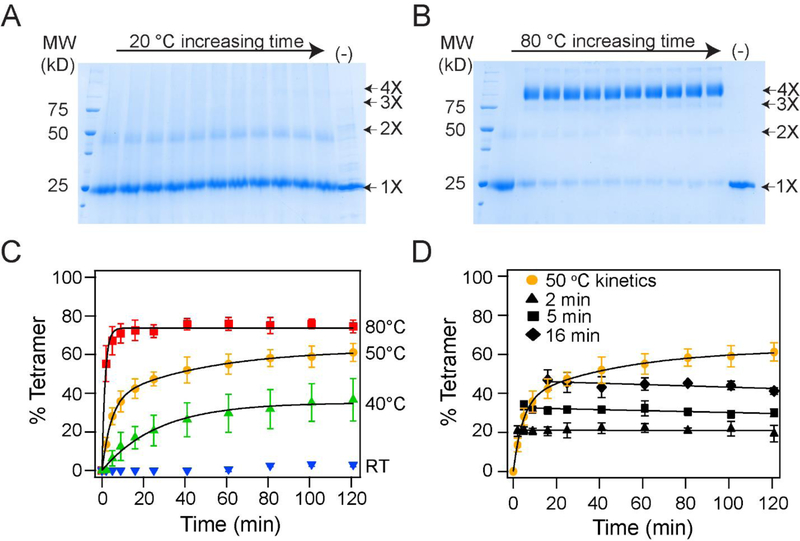
Figure 2. In vitro folding of the KvAP channel is dependent on temperature.
(A, B) Time course of the in vitro folding of the KvAP channel at 20 °C (A) and at 80 °C (B). Representative SDS-PAGE gels showing glutaraldehyde crosslinking of the in vitro folding reaction at various time points over 2 hours is shown. The last lane is a time point at 2 hours without glutaraldehyde added (−). The oligomeric nature of the crosslinked band is indicated. (C) Tetramerization kinetics of the KvAP channel at different temperatures are plotted. Data have been fitted with either a single (40 °C and 80 °C) or a double exponential (50 °C). Data at 20 °C could not be fitted to an exponential. (D) Temperature downshift arrests folding of KvAP. The in vitro folding reaction for the KvAP channel was carried out at 50 °C. After incubation for 2, 5 or 16 minutes, the samples were transferred from 50 °C to 20 °C and the tetramerization kinetics were followed. No additional tetramerization was observed at 20 °C. The data points following the downshift are fitted with a straight line. Error bars shown in C and D are standard deviations for n =3 using at least 2 independent protein preparations.