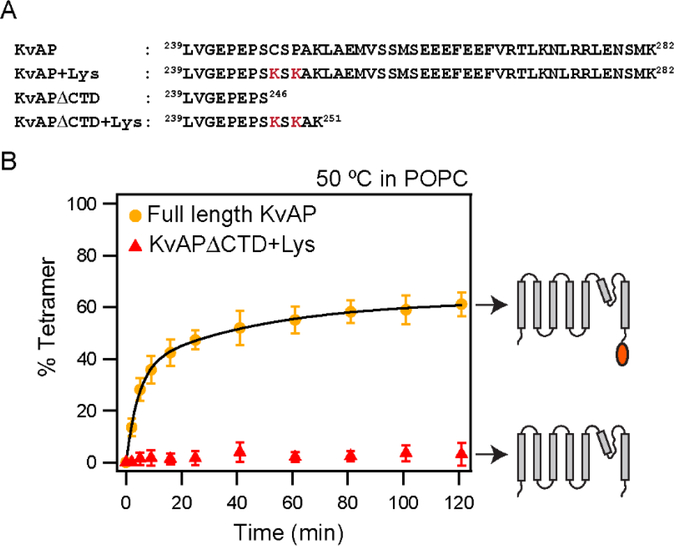
Figure 5. Deletion of the CTD abolishes tetramerization of KvAP in POPC lipids.
(A) The C-terminal sequences of the KvAP channel constructs used are shown. All constructs contain the wild type KvAP channel residues 1 – 238. The S6 transmembrane segment in the KvAP channel ends at residue 237. The Lys residues introduced to enhance glutaraldehyde crosslinking are indicated in red. (B) Time course of in vitro folding of the KvAPΔCTD+ Lys channel and the full length control in POPC lipid vesicles at 50 °C. Shown to the right are schematic illustrations of the constructs used. Data for the full-length KvAP channel is from Figure 2. Tetramerization kinetics were quantitated from n=3 experiments with at least 2 protein preparations. Error bars are standard deviations. Tetramerization kinetics for KvAPΔCTD+Lys could not be fitted with an exponential equation.