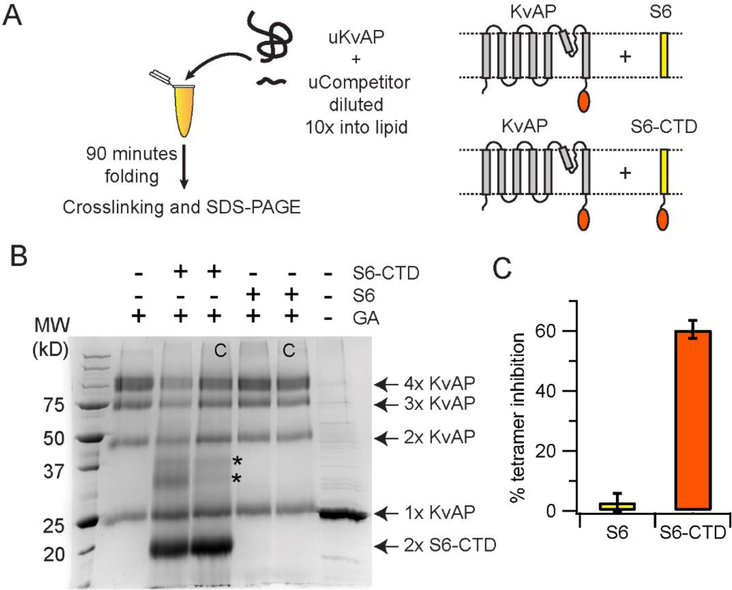
Figure 7. The CTD inhibits in vitro folding of KvAP.
(A) The reaction scheme used in the competition assay is shown. The competitors used are the S6-CTD or the S6 peptide. The schematic representation of the proteins used in the assay are shown. (B) SDS-PAGE gel showing the glutaraldehyde crosslinking of the KvAP folding reaction carried out in the presence of the S6+CTD or the S6 peptide. The identity of the protein bands observed on glutaraldehyde crosslinking are indicated. The protein bands indicated by the asterisk potentially corresponds to a KvAP+S6-CTD dimer and a S6-CTD tetramer based on their molecular weights. Control lanes where the competitor was added after completion of folding are marked with a C. A five-fold molar ratio of competitor to unfolded KvAP is used in all lanes. Monomers of S6-CTD and the S6 peptide are not visible on this gel because of their low molecular weight. A decrease in the density of the KvAP tetramer band is seen in the case of folding in the presence of S6-CTD but not in the presence of the S6 peptide. (C) Inhibition of the in vitro folding reaction. Bar graph showing the % of tetramer inhibition observed in the presence of the S6+CTD and the S6 peptide. Error bars correspond to the standard deviation for n = 3 from at least 2 protein preparations.