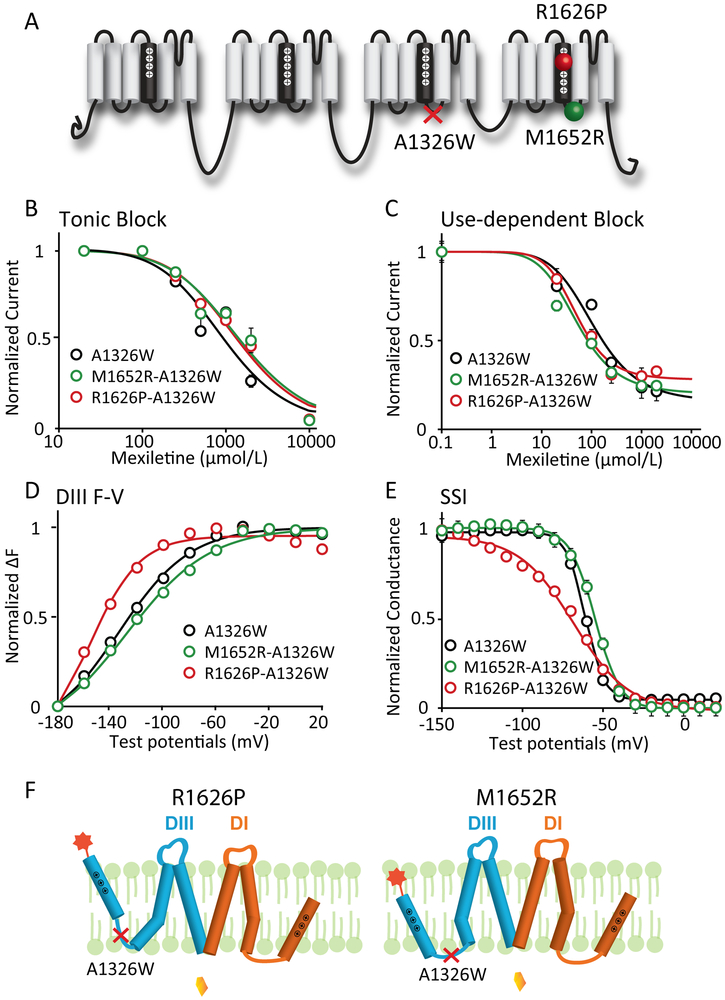
Figure 3: Mutation that decouples the DIII-VSD from DIII-pore eliminates differences in mexiletine sensitivity among LQT3 variants.
A. Locations of the decoupling mutation A1326W and two LQT3 variant mutations, R1626P and M1652R. A1326W resides on the S4-S5 linker of DIII, a motif that is known to regulate energetic coupling between the VSD and pore.
B. Concentration dependence of TB for A1326W, M1652R-A1326W, and R1626P-A1326W channels (n=3 for each drug condition). EC50 values were 965 μM for WT, 1562 μM for M1652R, and 1441 μM for R1626P channels.
C. Concentration dependence of UDB for M1652R-A1326W, and R1626P-A1326W channels (n=3 tested for each drug condition). EC50 values were 113 μM for WT, 51 μM for M1652R, and 52 μM for R1626P channels.
D. Voltage dependence of steady-state fluorescence of DIII for A1326W, M1652R-A1326W, and R1626P-A1326W channels (n=4 tested for each variant). The differences in voltage dependence of DIII-VSD activation is similar with the A1326W as a background mutation. DIII F-V curve of M1652R-A1326W still showed depolarizing shift, while R1626P-A1326W showed hyperpolarizing shift compared to A1326W channels.
E. Steady-state inactivation (SSI) curves of WT, R1626P, and M1652R channels (n=4 tested for each variant). The differences in SSI among different mutations are also preserved in presence of the A1326W background mutation.
F. Proposed schematic showing a model of how A1326W eliminates the different sensitivities among LQT variants.