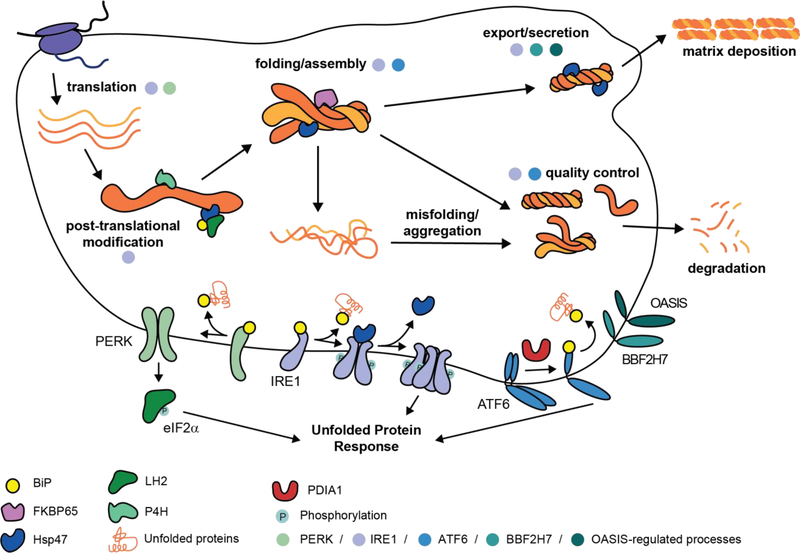
Figure 1 |. Collagen production.
Nascent procollagen polypeptides, comprised of N-propeptide (~15 kDa), triple-helical (up to ~100 kDa), and C-propeptide (~30 kDa) domains, are first co-translationally imported into the endoplasmic reticulum (ER). Within the ER, they undergo extensive co- and post-translational modifications prior to folding. These modifications include introduction of an N-glycan in the C-propeptide domain, extensive hydroxylation of proline residues that is required for thermal stability, and lysine hydroxylation that promotes extracellular crosslinking. Procollagen folding in the ER begins with the autonomous folding of the ~30 kDa C-propeptide domain on each monomeric strand, which can only begin after the entire protein is translated. The C-propeptide is a cysteine-rich domain whose folded state is stabilized by multiple intramolecular disulfide bonds. After the C-propeptide folds, individual C-propeptide domains recognize each other and assemble, a process that, at least for the fibrillar collagens, is mediated by Ca2+- and intermolecular disulfide bonds [62]. The resulting assembled C-propeptide trimer controls the triple helix register and initiates zipper-like folding of the proline- and glycine-rich triple-helical domain, a process that itself requires isomerization of hundreds of proline peptide bonds to the trans configuration. Triple-helix formation attenuates further procollagen hydroxylation, and sets the stage for secretion of the protein via a non-canonical pathway. For the fibrillar collagens, the mature protein is produced by cleavage of the propeptide domains, initiating extensive supramolecular assembly and the generation of hierarchical tissue architectures. This process is orchestrated by an extensive suite of ER chaperones and quality control mechanisms that are regulated by the three arms of the unfolded protein response (IRE1, ATF6, and PERK), as well as the related transcriptional responders OASIS and BBF2H7, which are highlighted in the lower portion of the figure.