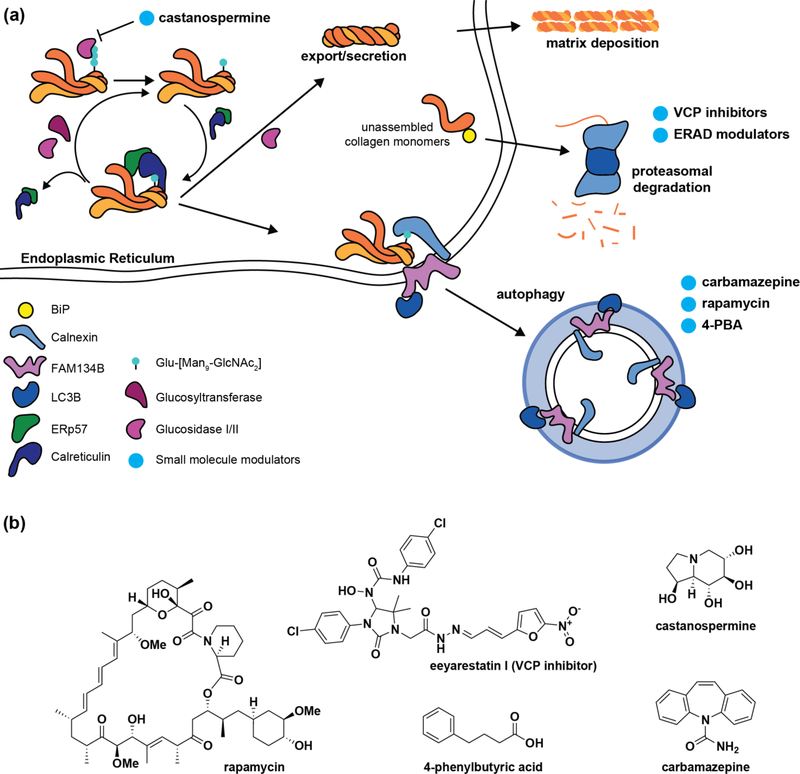
Figure 3 |. Procollagen quality control.
(a) While folding-competent procollagen molecules exit folding and modification cycles for secretion, slow-folding or aggregated procollagen is recognized by calnexin and targeted to autophagy, at least in part by the ER-phagy receptor FAM134B. Meanwhile, unassembled and/or misfolding procollagen monomers can be targeted to the proteasome via ER-associated degradation (ERAD), a process mediated by the transitional endoplasmic reticulum ATPase VCP. Reducing degradation of procollagen chains by using VCP inhibitors to prevent ERAD targeting or treatment with the calnexin cycle entry inhibitor castanospermine could prove beneficial for collagenopathies stemming from hyperactive quality control. In other cases, the persistence of misfolded collagen aggregates can overwhelm cellular clearance pathways, leading to escape of misfolded collagen molecules or stress-induced cellular dysfunction. Treatment with the autophagy activators carbamazepine, rapamycin, and 4-PBA may hold promise in reducing the disease burden of specific collagen variants that accumulate inside cells. (b) Structures of small molecule modulators highlighted in (a).