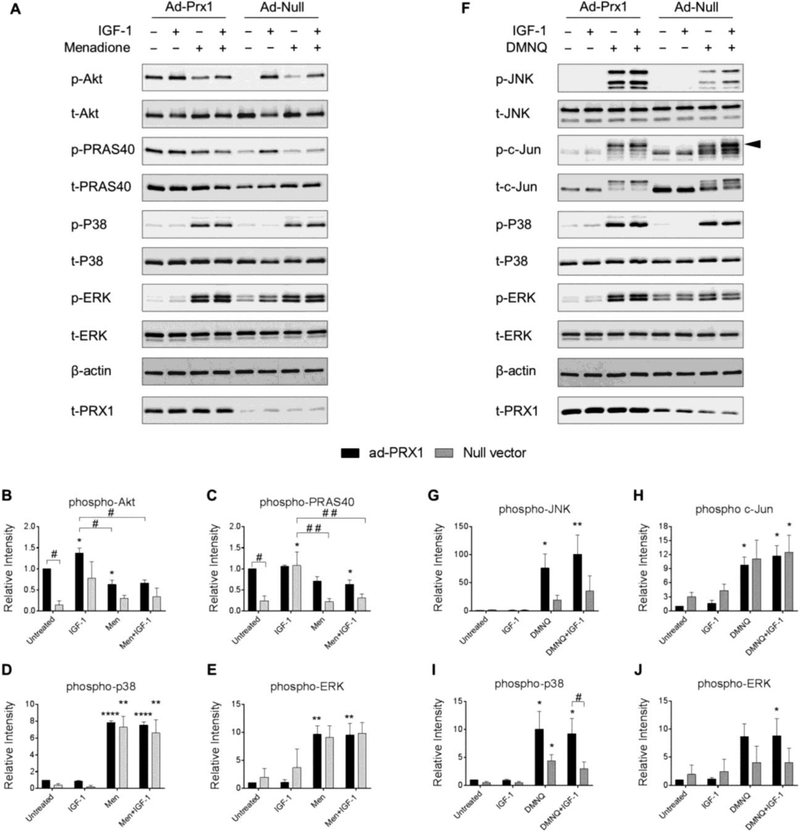
Figure 6. Effect of adenoviral overexpression of Prx1 on IGF-1 and MAP kinase signaling with and without menadione and DMNQ.
Chondrocytes were transduced with adenoviral vectors encoding Prx1 or a null empty vector control and were treated with IGF-1 alone (50 ng/ml), menadione (25 μM), DMNQ (25 μM) or pre-treated with menadione (25 μM) or DMNQ (25 μM) for 30 min prior to IGF-1 treatment for 60 min. Cell lysates were immunoblotted with antibodies to the indicated proteins pertinent to IGF-1 and MAP kinase signaling cascades. (A) Representative immunoblots from 3 independent experiments (n=3) showing the effect of Prx1 overexpression on menadione-induced phosphorylation of Akt, PRAS40, p38, and ERK. (B-E) Results of densitometric analysis showing the effect of Prx1 overexpression on phosphorylation of Akt, PRAS40, p38, and ERK in response to menadione treatment. (F) Representative immunoblots from 4 independent experiments (n=4) showing the effect of Prx1 overexpression on DMNQ-induced phosphorylation of JNK, c-Jun, p38, and ERK. (G-J) Results of densitometric analysis showing the effect of Prx1 overexpression on phosphorylation of JNK, c-Jun, p38, and ERK in response to DMNQ treatment. Phospho-proteins are normalized to respective total protein. For c-Jun, β-actin was used as a loading control. Arrow on c-Jun blots indicates electrophoretic shift of the c-Jun monomer which is indicative of serine and threonine phosphorylation (maximal phosphorylation). Data are mean±SEM expressed as relative intensity compared to ad-Prx1 control Asterisks represent significant differences compared to control (*p<0.05, **p<0.01, ****p<0.0001). # represent significant differences between highlighted conditions (# p<0.05, # # p<0.01) (ANOVA).