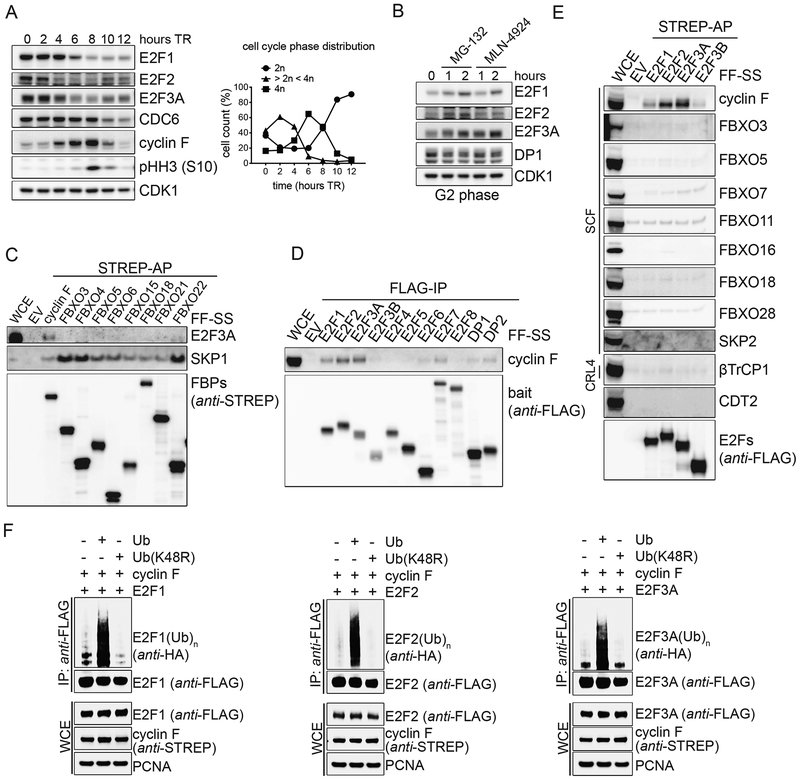
Figure 1. E2F1, E2F2, and E2F3A bind to and are ubiquitylated by cyclin F.
A. RPE-1 cells were synchronized in G1/S by a thymidine block and then released in fresh medium for the indicated hours. Cells were collected and processed for FACS analysis (Fig. S1A), and Western blot. The graph on the right summarizes the results of the FACS analysis. TR, thymidine block and release.
B. RPE-1 cells were synchronized in G2 by a thymidine block followed by a release in fresh medium for 8 hours, and treated with either MG-132 or MLN-4924 for the indicated hours. Cells were then collected, lysed and immunoblotted.
C. HEK-293T cells were transfected with an empty vector (EV) or the indicated STREP-tagged F-box protein constructs (FBPs). Whole cell extracts (WCEs) were subjected to affinity-purification (AP) with Streptavidin resin and immunoblotted.
D. HEK-293T cells were transfected with an EV or constructs expressing FLAG-tagged members of the E2F transcription factor family and their hetero-dimerization partners. WCEs were subjected to immune-purification (IP) with an anti-FLAG resin and immunoblotted.
E. The experiment was performed as in (C), except that HEK-293T cells were transfected with the indicated constructs.
F. HEK-293T cells were co-transfected with FLAG-tagged activator E2Fs, STREP-tagged cyclin F, and either HA-tagged Ubiquitin or Ubiquitin(K48R) mutant. WCEs were subjected to IP with an anti-FLAG resin under denaturing conditions and immunoblotted.