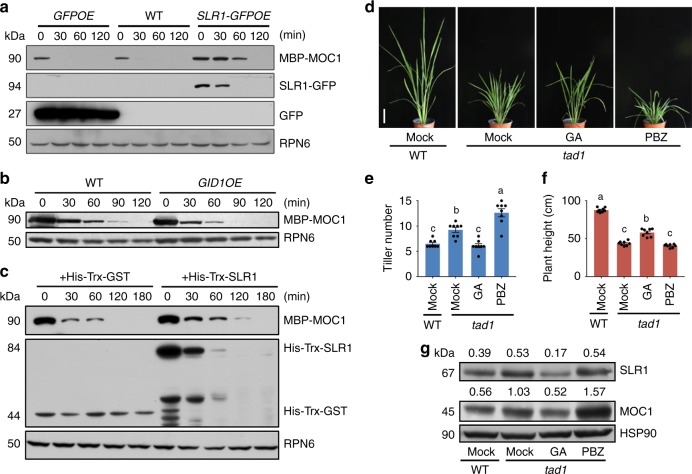
Fig. 4.
SLR1 inhibits the degradation of MOC1 independently of TAD1. a In vitro cell-free protein degradation assay, showing degradation of MBP-MOC1 in extracts from WT, GFPOE and SLR1-GFPOE transgenic plants. Immunoblots were probed with anti-MBP (α-MBP). Ribosomal protein 6 (RPN6), loading control. b In vitro cell-free protein degradation assay, showing degradation of MBP-MOC1 in extracts from WT and GID1OE transgenic plants. Immunoblots were probed as in (a). c In vitro cell-free protein degradation assay, showing that degradation of MBP-MOC1 was inhibited by His-Trx-SLR1, but not His-Trx-GST. Immunoblots were probed as in (a). d Effects of GA and PBZ treatments on tad1. Scale bar, 10 cm. e, f Quantification of the tiller number (e) and height (f) of plants shown in (d). Different lowercase letters indicate significant differences (Tukey’s HSD test, p < 0.01; mean ± s.e.m., n = 8). g Immunoblot analysis of SLR1 and MOC1 proteins in shoot basal tissues (0–0.5 cm) after treatments. Tissue samples were analyzed by protein blotting using α-SLR1 and α-MOC1. HSP90, loading control. Values above panels indicate signal strength for SLR1 and MOC1 in arbitrary units determined by densitometry. The full scans of immunoblots are shown in Supplementary Fig. 12. Source data are provided as a Source Data file