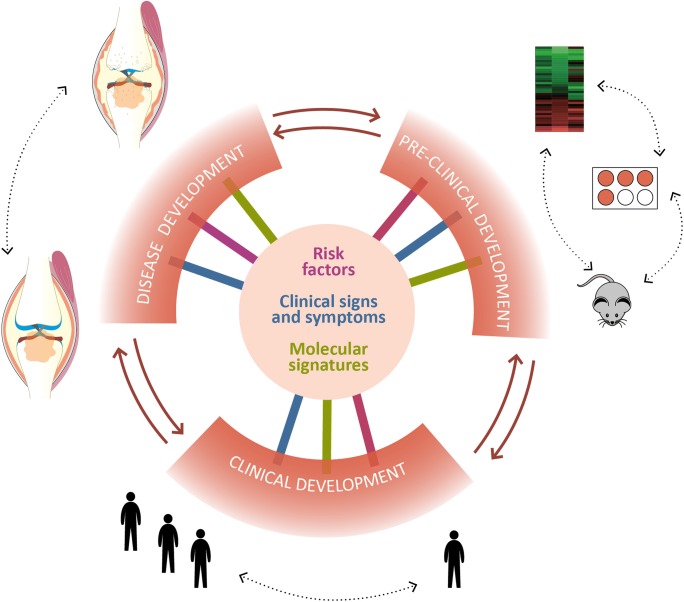
Fig. 3.
Integration of OA stratification categories with the translational cycle. The key stratification categories should be applied to studies of disease development and to pre-clinical and clinical drug development pathways. To refine critical stratification measures and identify relevant end points for pre-clinical and clinical studies, nextgeneration sequencing, “omics”, and cytometry approaches should be integrated with risk factors, clinical signs and symptoms. Tissue-based end points and stratification measures should be derived using well-phenotyped healthy and diseased tissues from the joint. Where appropriate, embedding tissue collection and analysis within enrolment and outcome stages of clinical trials would inform future studies across the translational cycle