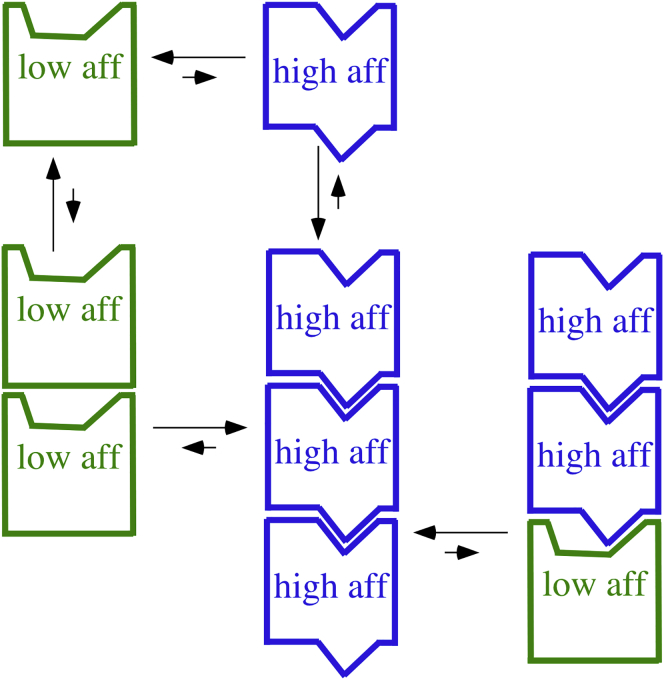
Figure 4.
A diagram of the high- and low-affinity conformations. For monomeric subunits, the high-affinity conformation is highly disfavored, and the top and bottom surfaces form a poorly matched interface. In the high-affinity conformation, the top and bottom surfaces are rearranged so that they form a large and snugly fitting interface. The extra bond energy of this interface is sufficient to compensate for the free energy needed to switch to high affinity, so polymerization favors the switch to high affinity. The right-hand PF shows the process of elongation, in which a subunit is added in the low-affinity conformation and switches to high affinity. The high-affinity conformation involves internal rotations of the subdomains and movement of helix H7. These are not shown because the important switch is in the top and bottom interface surfaces. For simplicity, the diagram shows monomeric subunits, applicable to FtsZ. For dimeric tubulin, the conformational change would be transmitted across both α and β subunits, consistent with crystallography. To see this figure in color, go online.