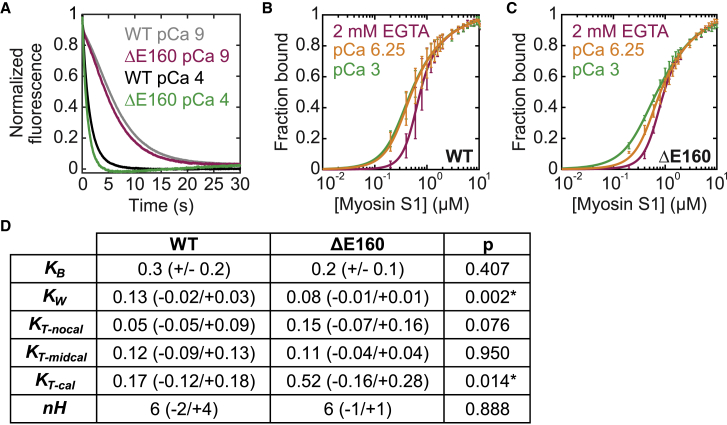
Figure 4.
Effects of the ΔE160 mutation in troponin T on thin-filament regulation. (A) Normalized stopped-flow fluorescence traces of myosin binding to RTFs. The pyrene fluorescence is quenched at a higher rate at high calcium (pCa 4, green/black) than at low calcium (pCa 9, magenta/gray). The traces for WT (black/gray; average of n = 6 curves each) and the ΔE160 mutant (magenta/green; average of n = 4 curves each) are similar at each calcium concentration. (B and C) Steady-state titrations of RTFs with myosin for the WT (B) or mutant (C) protein conducted at three distinct calcium concentrations: pCa 3 (cal, green), pCa 6.25 (midcal, orange), and 2 mM EGTA (nocal, magenta). Curves are fits to the data. Error bars show the SD of five technical replicates. (D) A table of parameter values obtained for WT and ΔE160 troponin complexes from stopped-flow measurements (for KB) and using the computational tool (for all others) is given. Values in parentheses indicate the SD of six (WT) or four (ΔE160) replicates for KB and 95% confidence intervals determined using the computational tool for all other parameters. Asterisks indicate statistical significance at the 95% confidence level. To see this figure in color, go online.