Figure 2.
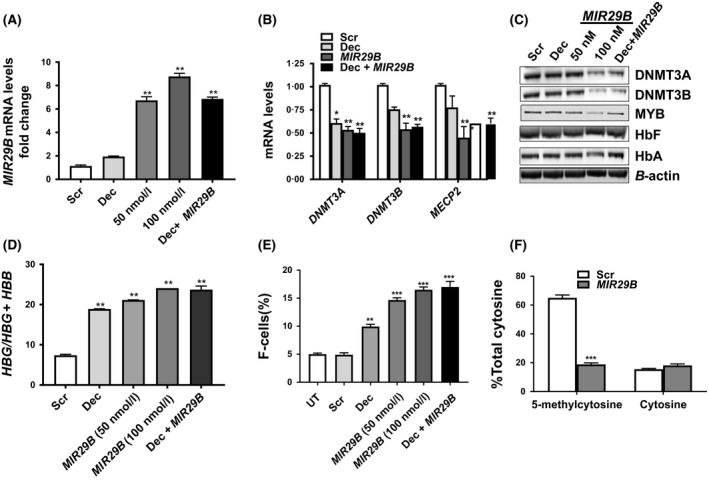
MIR29B regulates HBG gene expression through proximal promoter 5‐hydroxymethylation in human primary erythroid progenitors. (A) Shown is the fold change of MIR29B expression by reverse transcription‐quantitative polymerase chain reaction (RT‐qPCR) in erythroid progenitors generated from CD34+ stem cells (see Material and Methods), treated with Scr (100 nmol/l), MIR29B (50 or 100 nmol/l) or 0·5 μmol/l Dec alone or combined treatment with 0·5 μmol/l Dec and MIR29B 100 nmol/l. The expression of MIR29B in Scr control was set to one after normalization to the SNORD48 endogenous control. The data are shown as the mean ± SEM. (B) Shown is the fold change of DNMT3A,DNMT3B, and MECP2 mRNA levels in erythroid progenitors under the various conditions normalized to the internal control β‐actin by RT‐qPCR analysis. (C) Western blot analysis of the various proteins shown in erythroid progenitors for the different treatment conditions. (D) The mRNA levels of HBG and HBB in erythroid progenitors was calculated as the ratio: HBG/HBG + HBB for the different conditions by RT‐qPCR. (E) Flow cytometry analysis was completed to determine F‐cell levels in erythroid progenitors stained with fluorescein isothiocyanate‐labelledanti‐HbF antibody under the different treatment conditions. (F) Levels of 5‐methylcytosine and unmodified cytosine (cytosine) at the HBG promoter −54 CCGG site for Scr control and MIR29B transfected erythroid progenitor cells. Scr, scramble; UT, untreated. *P < 0·05, **P < 0·01, ***P < 0·005
