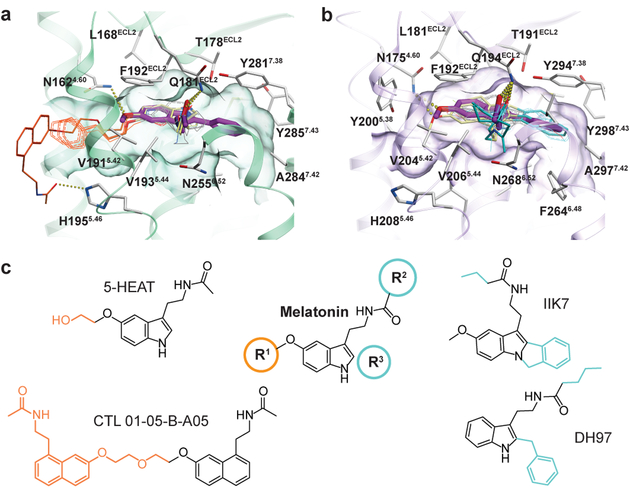
Fig. 3 |. Selectivity determinants of ligands at MT1 and MT2.
a, Docking of selective ligands into MT1 (green), with 2-pmt (purple) from the crystal structure shown as reference. Ligands selective for MT1 (compounds 63, 64, 65a, and 65b)22 are shown in grey. Two representative ligands, 5-HEAT16 and CTL 01–05-B-A058 are coloured pale yellow, with their selectivity-conferring substituents (R1 position) shown in orange. b, Docking of ligands into MT2 (violet), with 2-pmt (purple) shown as reference. Non-selective (tasimelteon, TIK30122) and selective (UCM1014, K185, and 4P-PDOT)22 ligands are shown in grey. Two representative ligands, DH9717 and IIK717 are coloured pale yellow, with selectivity-conferring substituents (R2 and R3 positions) shown in cyan. Predicted hydrogen bonds are shown as dotted lines in a and b. c, Melatonin SAR, where R1 substituents confer MT1 selectivity (orange), and substituents in R2 and R3 positions confer MT2 selectivity (cyan). See Supplementary Table 1 for a list of all docked ligands.