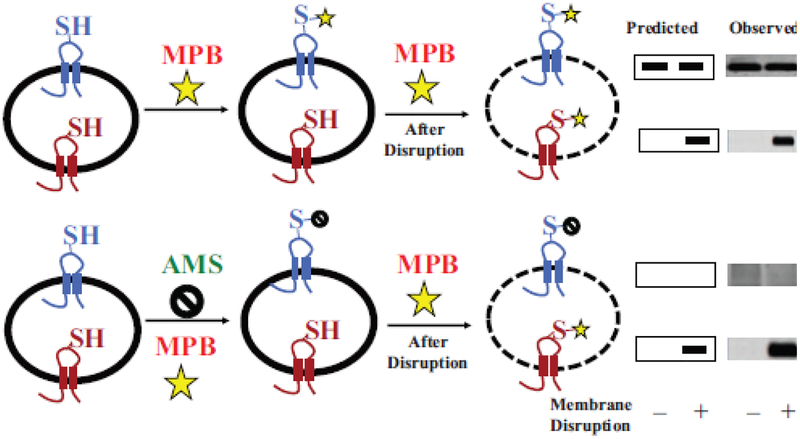
Figure 4.
General strategy for SCAM™ using impermeable thiol reagents. A target membrane protein containing a single cysteine replacement exposed either to the exterior (blue) or interior (red) side of a cell membrane, membrane vesicle or proteoliposome is shown. Half of the sample is treated with the detectable thiol reagent 3-(N-maleimidylpropionyl) biocytin (MPB) to specifically label the externally exposed cysteine (top panel), and the other half is treated with the non-detectable thiol reagent 4-acetamido-4’-maleimidylstilbene-2,2’-disulfonic acid (AMS) (bottom panel) to protect external cysteines during subsequent MPB lableing. Both halves are either kept intact (–) or disrupted (+) by sonication or detergent treatment to expose the interior cysteine followed by treatment with MPB to specifically label the previously inaccessible internal cysteine residue. Labeling by MPB that can be blocked completely by pretreatment with AMS is an independent verification of an outside facing residue (bottom panel). Labeling by MPB that cannot be blocked by such AMS treatment is an independent verification of a residue that is facing the cytoplasm. The target protein is immunoprecipitated and resolved by sodium dodecyl sulfate-polyacrylamine gel electrophoresis and biotinylated protein is detected using avidin-horse radish peroxidase with the predicted and observed results shown on the right. A protein with dual topology would display an increase in the amount of labeled protein after disruption in the upper panel and reduced amount of labeling detected after disruption in the lower panel. The modified figure and legend were reprinted by permission from Springer Nature: [49] Copyright 2019.