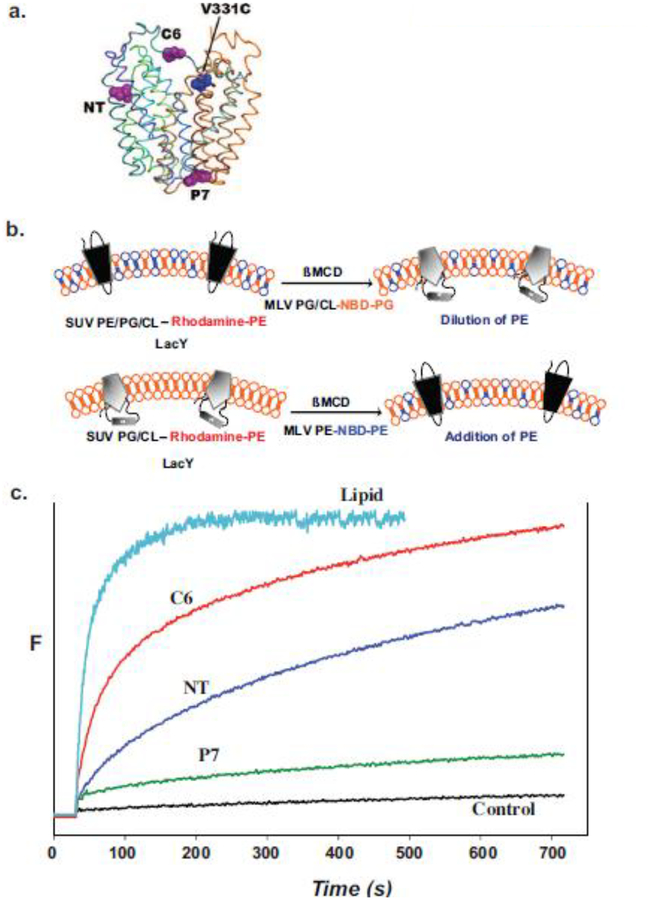
Figure 5.
Monitoring lipid-dependent topological changes in fliposomes. a. Schematic native structure of LacY showing the position of an engineered tryptophan residue in either EMD NT (residue 14), C6 (residue 205) or P7 (residue 250) relative to the chromophore at position V331C. b. LacY engineered to display high FRET intensity in the native conformation (upper left) or low FRET intensity in the inverted conformation (lower left) was reconstituted into small unilamellar vesicles (SUV) with or without PE, respectively. The SUVs contained trace amounts rhodamine labeled PE. Multilamellar vesicles (MLV) containing PG and CL with a trace amount of 6-[(7-nitro-2–1,3-benzoxadiazol-4-yl)amino]hexanoyl-PG (NBD-PG) or PE with a trace amount of NBD-PE were added to the SUVs containing native or inverted LacY, respectively. Transfer of lipids between SUVs and MLVs was initiated by addition of ß-methyl cyclodextrin (ßMCD)-loaded MLVs to the SUV suspension. The rate of lipid transfer was monitored by FRET between rhodamine- and NBD-labeled lipids. The rate of LacY topological change was monitored by FRET between a tryptophan residue and a chromophore in the C-terminal six TMD bundle of LacY. c. Time scale for change in FRET upon addition of PE to proteoliposomes containing LacY in the absence of PE. Control indicates lack of changes in FRET when SUVs and MLVs both lack PE. Figure 5b was reproduced from[98] by permission of the National Academy of Sciences, USA. Figure 5c was constructed based on data presented in [98].