Fig. 2. Glyan dependent quality control.
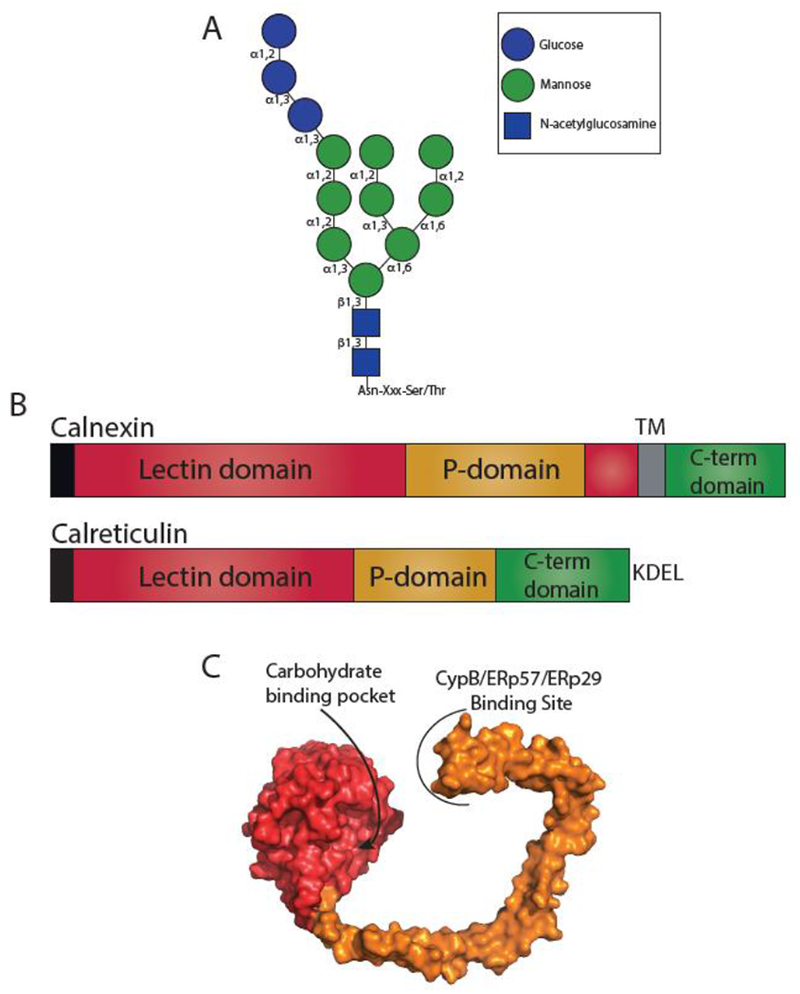
(A) Structure of an N-linked glycan. N-linked glycans are transferred en bloc to an Asn residue in acceptor sites Asn-X-Ser/Thr, where X is not a proline. Glycosidic bonds are denoted. (B) The domain architecture of calnexin and calreticulin. Both calnexin and calreticulin possess an N-terminal signal sequence (black) that is cleaved in the mature protein. Calnexin possesses a lectin domain (red) that is composed of two regions separated by the P-domain (orange), a transmembrane region (TM) (grey) and a cytosolic C-terminal domain (green). Calreticulin possesses a contiguous lectin domain, a P-domain, a C-terminal domain, and a KDEL retention motif. (C) Surface representation of the crystal structure of the luminal domain of calnexin (PDB: 1JHN). The lectin domain is shown in red and the P-domain in orange. The carbohydrate binding pocket in the lectin domain and the binding site of CypB/ERp57/ERp29 on the tip of the P-domain are designated.
