Figure 1.
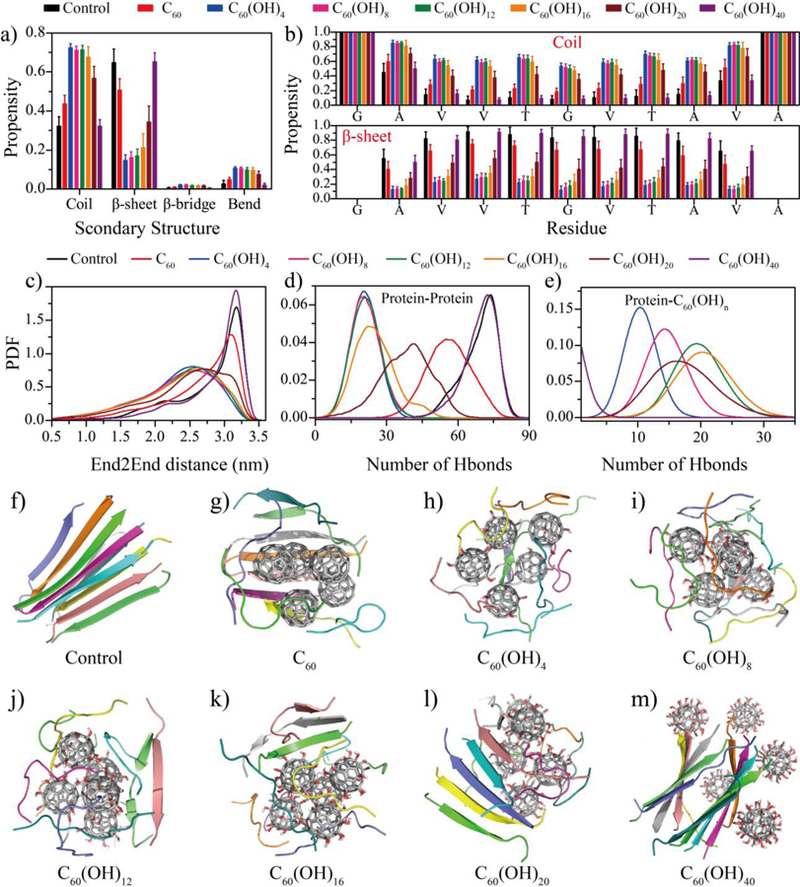
Inhibition of NACore β-sheet formation in silico. (a) The averaged content of secondary structures including coils, β-sheets, β-bridges and bends from equilibrium simulations. (b) The propensity of each amino acid to form coil and β-sheet conformation. The probability distribution function (PDF) of (c) the end-to-end distance (End2End), (d) the number of inter-chain hydrogen bonds (Hbond), and (e) the number of hydrogen bonds formed with nanoparticles for each peptide. (f-m) Representative snapshot structures of each simulated molecular systems, randomly selected from 20 independent simulation trajectories.
