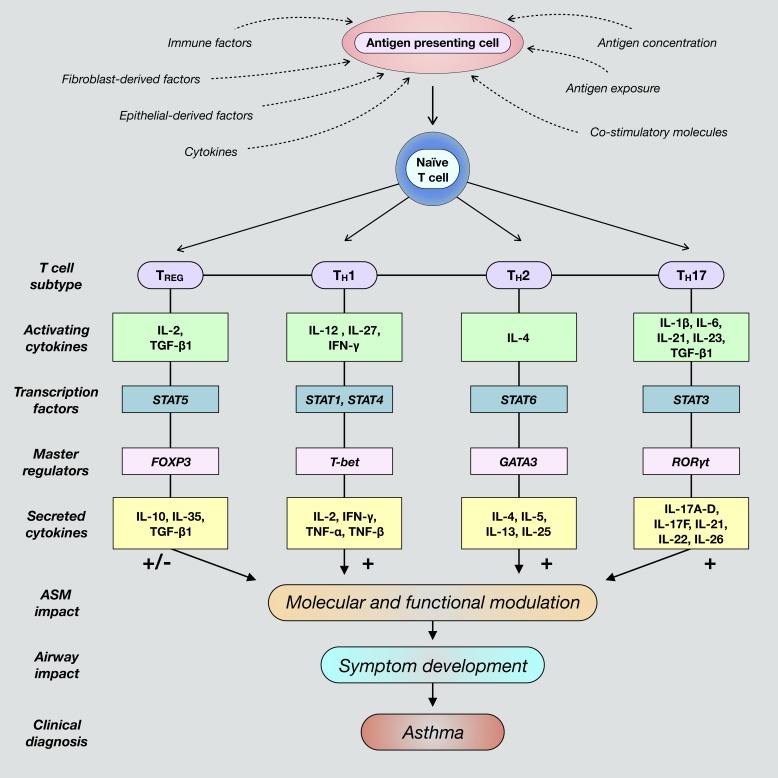
Fig. 1.
Differentiation of naïve cluster of differentiation 4-positive (CD4+) T cells toward polarized, individual effector T cell fates is determined by the presence of unique activating factors and molecules present within a given inflammatory airway microenvironment. These commonly include various factors secreted by epithelial cells and fibroblasts, such as cytokines and chemokines. Additionally, T cell differentiation is promoted when antigen-presenting cells are exposed to foreign antigens and subsequently interact with naïve T cells. Classical T cell subtypes involved in airway immunity include regulatory T (TREG), T helper 1 (TH1), TH2, and TH17 cells. Mature T cells are characterized by the expression and activation of subtype-specific transcription factors and master regulators that ultimately induce secretion of unique cytokines contingent upon differentiation fate. These cytokines activate cognate receptors expressed by structural airway cells that direct immune responses and contribute to dysregulated airway smooth muscle (ASM) function and asthma pathogenesis. FOXP3, forkhead box protein P3; GATA3, trans-acting T cell-specific transcription factor GATA-3; RORγt, retinoic acid receptor (RAR)-related orphan receptor-γ, thymus; T-bet, T cell-specific T-box transcription factor T-bet; TGF-β1, transforming growth factor-β1.