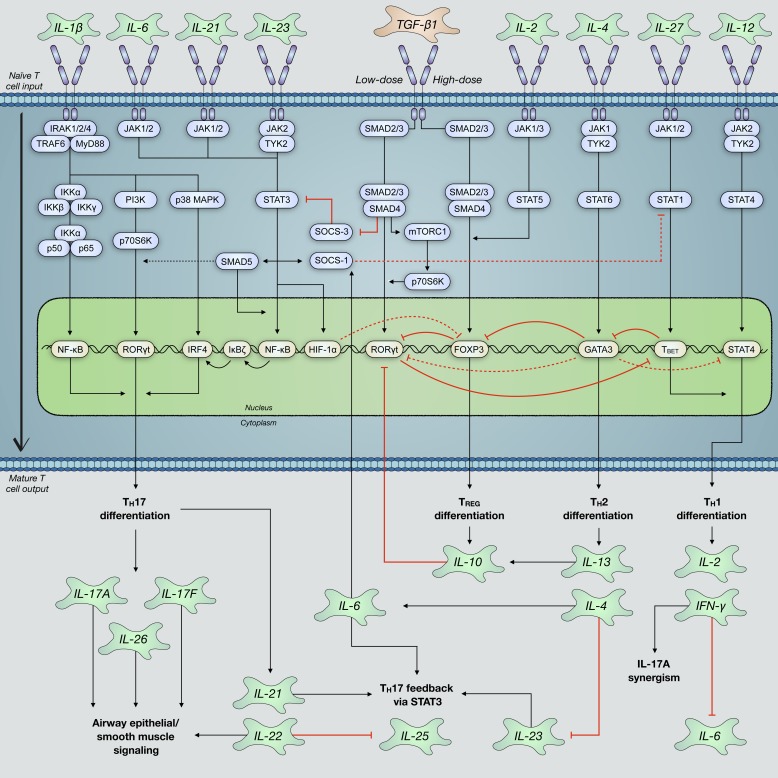
Fig. 4.
T helper 17 (TH17) cell differentiation relies on the coordination of several well-characterized cytokines and their activation of cognate receptors on the surface of naïve T cells. IL-6 and transforming growth factor-β1 (TGF-β1) are the most prominent drivers of TH17 polarization via canonical signal transducer mothers against decapentaplegic homolog (SMAD) and signal transducer and activator of transcription 3 (STAT3) pathways. This enhances activation of TH17-necessary transcription factor retinoic acid receptor (RAR)-related orphan receptor-γ, thymus (RORγt). Other cytokines, including IL-1β, IL-21, and IL-23, can further drive TH17 differentiation and, moreover, promote their sustained maintenance. Regulatory T (TREG)-, TH1-, and TH2-associated cytokines typically activate signaling pathways that repress RORγt activation and inhibit TH17 differentiation. However, depending on the precise composition of cytokines exposed to naïve T cell surface receptors and interactions between intracellular signaling pathways, there is evidence of increased TH17 differentiation by these cytokines. FOXP3, forkhead box protein P3; HIF-1α, hypoxia-inducible factor 1α; IRAK, IL-1 receptor-associated kinase; IRF-4, interferon regulatory factor 4; mTORC1, mammalian target of rapamycin complex 1; MyD88; myeloid differentiation primary response protein MyD88; p70S6K, ribosomal protein S6 kinase β-1; PI3K, phosphatidylinositol 4,5-bisphosphate 3-kinase; SOCS, suppressor of cytokine signaling; T-bet, T cell-specific T-box transcription factor T-bet; TRAF6, TNF receptor-associated factor 6; TYK2, nonreceptor tyrosine-protein kinase TYK2.