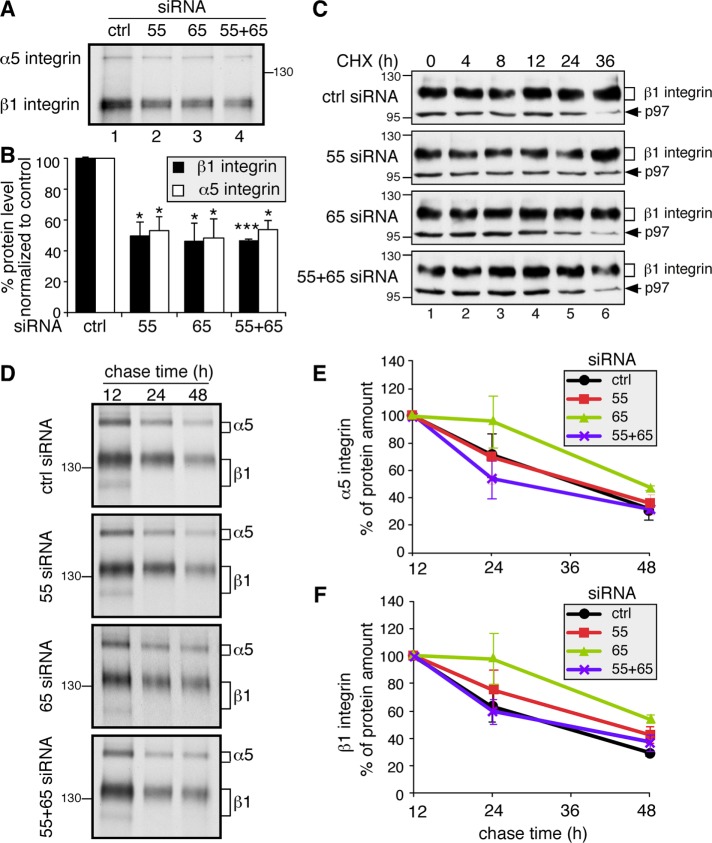
FIGURE 6:
GRASP depletion reduces α5- and β1-integrin synthesis but has no effect on their turnover. (A) GRASP depletion reduces protein synthesis of α5 and β1 integrin. An equal number of HeLa cells transfected with indicated siRNAs were labeled with Trans 35S-Label [35S] for 1 h. Immunoprecipitated α5β1 integrin was analyzed by gel electrophoresis and autoradiography. The protein synthesis rates of both α5 and β1 integrin decreased in GRASP-depleted cells. (B) Quantitation of A. Statistical analysis was performed by comparison with control (ctrl) siRNA-treated cells. *, p < 0.05; ***, p < 0.001. (C) Western blot of GRASP-depleted HeLa cells treated with CHX for the indicated times. At 72 h posttransfection with the indicated siRNA, cells were treated with 100 µM CHX for 0, 4, 8, 12, 24, and 36 h; lysed; and analyzed by Western blot for β1 integrin and p97 on the same gel. As GRASP knockdown cells have a lower level of integrins, we exposed those gels longer, so all cell lines had a similar signal at the 0 time point to start with, and the reduction of the protein was assessed over time. (D) GRASP depletion does not increase α5- and β1-integrin degradation. HeLa cells transfected with indicated siRNAs were labeled with Trans 35S-Label [35S] for 1 h and chased for 12, 24, and 48 h. Immunoprecipitated α5β1 integrins were analyzed by SDS–PAGE and autoradiography. (E) Quantification of α5 integrin in D. Note that there is no significant difference in the degradation rate of α5 integrin between control siRNA-treated and GRASP-depleted cells. (F) Quantification of β1 integrin in D. There is no significant difference in the degradation rate of β1 integrin between control siRNA-treated and GRASP-depleted cells.