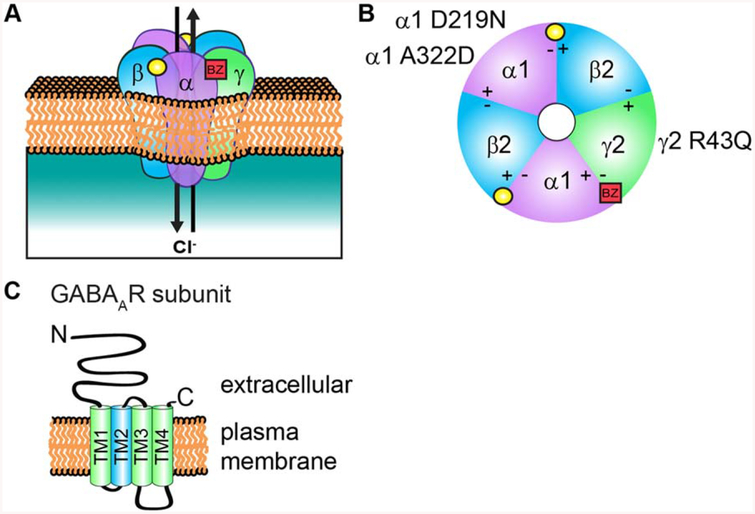
Figure 1.
GABAaR structure and subunit topology. (A) A representation of the heteropentameric GABAAR composed of αβγ subunits. Binding of the neurotransmitter GABA (yellow circle) at the αβ interface triggers ion channel opening and allows the rapid influx of Cl− and membrane hyper polarization in the mature nervous system. (B) Extracellular representation of the receptor showing all five subunits contributing to the central ion pore and the general binding sites of GABA (yellow circle) and BZs (red square). BZs bind at the interface of an α1/2/3/5 and y subunit. Also shown are specific mutations in a and γ2 subunits discussed in this review that contribute to epilepsy disorders by disrupting receptor assembly and trafficking. (C) All subunits have a common topology includ ing an extracellular N-terminal domain, short C-terminal tail, and four transmembrane domains (TM1–4). GABAAR subunit TM2 (blue) contributes to formation of the receptor ion channel pore, while the intracellular loop between TM3 and TM4 contain sites of phosphorylation and protein interactions that modulate channel function and/or trafficking. [Color figure can be viewed at wileyonlinelibrary.com]