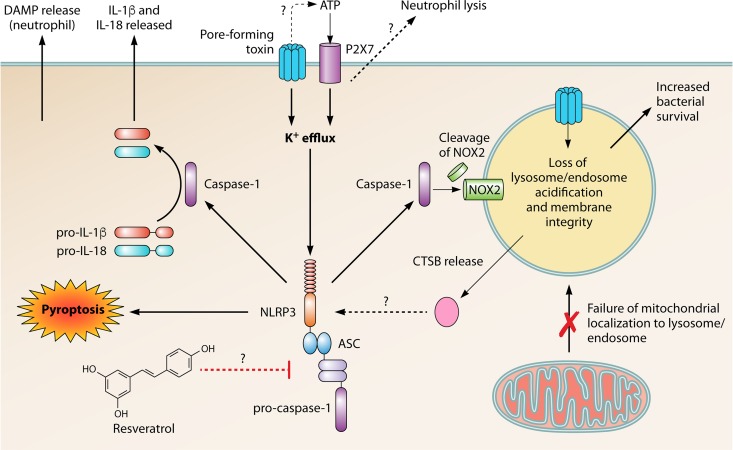
FIG 2.
Pore-forming toxins induce the NLRP3 inflammasome in phagocytes. Bacteria that encode pore-forming toxins can induce the NLRP3 inflammasome by forming pores within the membrane and facilitating potassium efflux. ATP, potentially leaving the cell via bacterial pores, binds to the purinoceptor P2X7 and induces K+ efflux that contributes to NLRP3 inflammasome activation and induces lysis in neutrophils through an unknown mechanism. Potassium efflux leads to oligomerization and activation of the NLRP3 inflammasome, consisting of the sensor protein NLRP3, adapter protein ASC, and pro-caspase-1, leading to cleavage and activation of effector caspase-1. Activation of caspase-1 leads to cleavage of pro-IL-1β and pro-IL-18 and subsequent release from the cell. Inflammasome activation can also lead execution of necrotic cell death via pyroptosis as well as DAMP release in neutrophils. Caspase-1 can also cleave components of the phagocyte NADPH oxidase, NOX2, which can lead to loss of endosomal acidification. Bacterial pores on the endosome also contribute to lack of acidification due to loss of membrane permeability as well as preventing the mitochondria from localizing with the endosome due to the strong activation of NLRP3. Loss of acidification leads to increased survival of bacteria within the phagosome/endosome. Cathepsin B (CTSB) can be released from damaged lysosomes and can also induce NLRP3 inflammasome activation, via an unknown mechanism. The antioxidant resveratrol can inhibit the expression and activation of the NLRP3 inflammasome, leading to a decrease in bacterial survival.