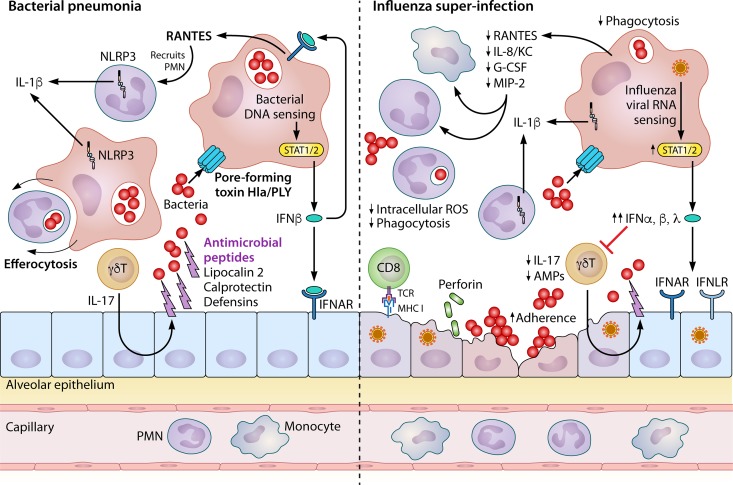
FIG 3.
Preceding influenza inhibits pulmonary immunity to bacterial pneumonia. Pulmonary innate immunity to bacteria (left) is orchestrated mainly by epithelial cells, macrophages, neutrophils, and gamma-delta T cells. The epithelium provides a physical barrier to infection and expresses antimicrobial peptides to kill extracellular bacteria. This expression is augmented by type 17 cytokines from gamma-delta T cells (γδT). Alveolar macrophages (AMs) patrolling the airspaces engulf bacteria through phagocytosis, eventually leading to killing in acidified phagosomes. Bacterial pore-forming toxins (notably Staphylococcus aureus Hla and Streptococcus pneumoniae PLY) allow the entry of bacterial DNA into the cytoplasm of alveolar macrophages, leading to interferon beta (IFN-β) production via STAT1/2 signaling. This IFN-β can bind receptors on epithelial cells but also signals in an autocrine fashion on these macrophages to induce production of RANTES and other chemokines. These chemokines recruit mainly neutrophils from the bloodstream, which can also phagocytose bacteria. Both neutrophils and macrophages contain the NLRP3 inflammasome, a scaffold of proteins serving to activate caspase-1 and other enzymes that cleave IL-1 cytokine family members (mainly IL-1β). Finally, macrophages prevent excess inflammation by engulfing dead or dying cells through a phagocytic process known as efferocytosis. Influenza induces susceptibility to bacterial infection through inhibiting antibacterial immune defenses (right). Influenza virus preferentially infects epithelial cells, leading to destruction of the epithelial barrier from viral infection and later cytotoxic CD8+ T cell activation. This increases the ability of bacteria to adhere to the epithelium. Production of both type I (α and β) and III (λ) IFNs is highly increased during the antiviral immune response, leading to inhibition of type 17 cytokines and antimicrobial peptide production. IL-1β production is also reduced by preceding influenza. Overall, chemokine production is reduced, while airspace cellularity is increased due to the immunopathologic neutrophil recruitment in response to influenza virus infection. Phagocytosis of bacteria by both macrophages and neutrophils is blunted, concomitant with a decrease in intracellular reactive oxygen species important for bacterial killing in the phagosome.