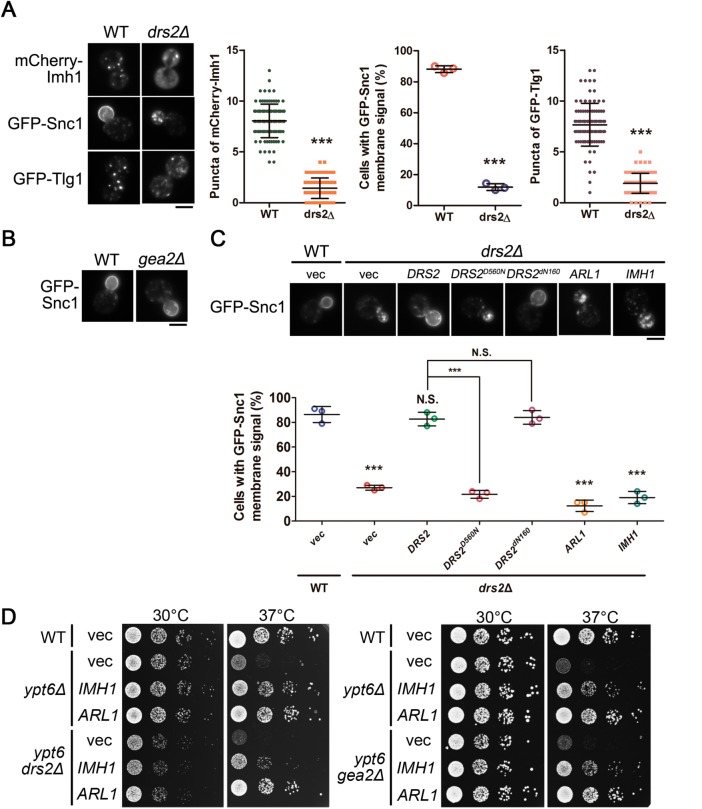
FIGURE 3:
The Arl1-Drs2-Gea2 ternary complex is not involved in the suppression of defects in endosome-to-TGN transport and temperature-sensitive growth in ypt6Δ cells. (A) Drs2 mediates the transport of Snc1 and Tlg1. Fluorescently tagged Imh1, Snc1, or Tlg1 was transformed into WT or drs2Δ cells (scale bar, 5 μm). Cells showing the GFP-Snc1 plasma membrane signal were quantified (n = 100) and the data are reported as the mean ± SD of three independent experiments (***p < 0.001; one-way ANOVA). Cells showing the mCherry-Imh1 or GFP-Tlg1 puncta were quantified (n = 100), and the data are reported as the mean ± SD of three independent experiments (***p < 0.001; paired t test). (B) Gea2 is not required for the proper transport of Snc1. GFP-Snc1 was transformed into WT or gea2Δ cells and examined by fluorescence microscopy (scale bar, 5 μm). (C) Arl1 is not involved in Drs2-mediated Snc1 transport. GFP-Snc1 was expressed in wild-type or coexpressed with Arl1, Imh1, or different forms of Drs2 in drs2Δ cells. Live cells were observed in mid–log phase by fluorescence microscopy. Cells showing the GFP-Snc1 plasma membrane signal were quantified (n = 100). The data are reported as the mean ± SD of three independent experiments (***p < 0.001; N.S. not significant; one-way ANOVA; scale bar, 5 μm). (D) The ternary complex of Arl1-Drs2-Gea2 is not involved in Arl1 and Imh1 suppressing temperature-sensitive growth defects in ypt6Δ cells. The indicated strains (wild-type, ypt6Δ, ypt6drs2Δ, ypt6gea2Δ) transformed with Arl1 or Imh1 were serially diluted 10-fold as indicated, spotted on plates, and incubated at 30 or 37°C.