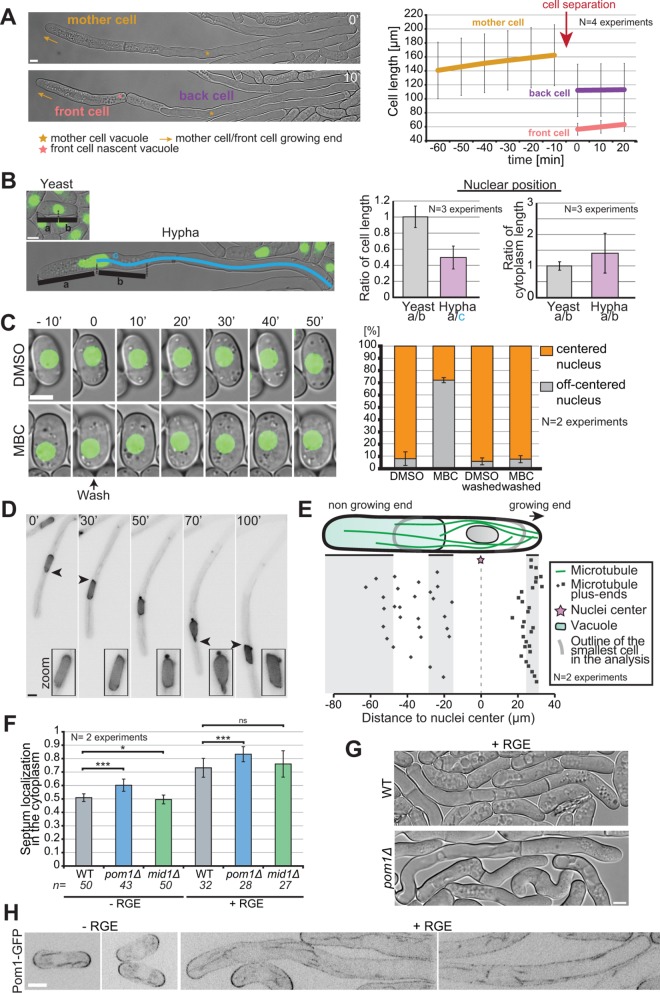
FIGURE 7:
Asymmetric cell division in S. japonicus. (A) Hyphae divide asymmetrically, giving rise to a front cell that retains the growing end but has to rebuild a vacuole and a back cell that retains the vacuole but has to rebuild a growing end (left). Hyphal cell length recording over time aligned on cell separation (right) (n = 20 hyphae). (B) Quantification of nuclear positioning in the cell and in the cytoplasm. Positioning was calculated through ratios as explained in the left panel (n > 50 cells per cell type). (C) Microtubules contribute to nuclear positioning. Cells were grown in microfluidic chambers for 3 h with DMSO or MBC and then washed for 50 min with EMM-ALU. Nuclear position was quantified before and after the wash in >65 cells per condition. Note that most nuclei recentered rapidly after washout, but we quantified after 50 min to include a few delayed cells. (D) Fluorescence images of a hypha expressing NLS-GFP showing an example of nuclear shape alteration over time. Arrowheads point to nuclear envelope protrusion indicative of exerted forces. Insets show zoom on the nucleus. (E) Schematic of the localization of microtubule plus ends in the hyphal form. Each dot represents a microtubule plus-end position. The nuclear position was used as a reference point in all measurements. Shaded areas show the range of positions of the cell front, vacuole front, and cell back (n = 50 microtubule tips in 10 hyphae). (F) Quantification of septum position in the cytoplasm of yeast and hyphae, in WT, pom1∆, and mid1∆ strains. ns: p = 0.22; *: p = 0.03; ***: p < 9.08 × 10–08; t test. (G) DIC images of septated WT and pom1Δ hyphae. Septation plane positioning in pom1Δ hyphae is biased toward the vacuole. (H) Middle plane fluorescence images of Pom1-GFP under inducing and noninducing conditions. In the hyphal form we show both the front and the back of cells. Scale bars: 5 µm.