Abstract
Background:
Different sedation strategies are used during endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA) for the diagnostic workup of lung cancer including general anesthesia (GA) and moderate sedation. However, no data are available about EBUS-TBNA under deep sedation (DS) with fiberoptic intubation directed by the investigator.
Materials and Methods:
A retrospective analysis of EBUS-TBNAs under GA (n = 160) or DS (n = 105) was performed.
Results:
Unadjusted diagnostic yield did not differ significantly between the groups (GA: 42.5% vs. DS: 53.3%; P = 0.1018). Similar results were obtained when only patients with a final diagnosis of malignancy were analyzed (GA: 53.6% vs. DS: 61.5%; P = 0.2675). Adverse events (AEs) occurred more often under DS (GA: 27.5% vs. DS: 59.1%; P < 0.0001) due to more sedation-related problems whereas severe AEs tended to be higher under GA (GA: 7.5% vs. DS: 1.9%; P = 0.0523).
Conclusion:
In summary, our data show that the diagnostic yield and the complication rate of EBUS-TBNA performed under DS are similar compared to GA. Hence, in an appropriate setting, EBUS-TBNA can be performed safely under DS.
Keywords: Bronchoscopy, complications, diagnostic yield, endobronchial ultrasound, sedation
INTRODUCTION
Endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA) has become one of the most important tools for the diagnostic workup of lung cancer, as it is an effective technique for the sampling of mediastinal lymph nodes with a low complication rate.[1,2] To facilitate the procedure and to increase patient tolerance, comfort, and cooperation, EBUS-TBNA is usually performed under sedation or general anesthesia (GA). Although a recent randomized trial demonstrated comparable diagnostic yields of EBUS-TBNA performed under moderate sedation (MS) versus GA, the optimal sedation strategy for EBUS-TBNA is still a matter of debate.[2,3,4] Cough suppression obtained by MS is often incomplete resulting in suboptimal conditions which might favor GA although access to GA for bronchoscopy is limited or unavailable in many institutions. A possible alternative could be deep sedation (DS) directed by the investigator with fiberoptic intubation under maintenance of spontaneous breathing as this approach has been found to be well tolerated and safe.[5,6,7] Due to the deeper level of sedation, DS might result in better cough suppression compared to MS improving patient tolerance and operating conditions. In addition, the placement of an endotracheal tube allows rapid retracting and advancement of the bronchoscope without removing the needle from the working channel. On the other hand, a deeper level of sedation in the absence of an anesthesiologist might increase the occurrence of complications. Therefore, the aim of this retrospective analysis was to compare diagnostic yield and complication rate of EBUS-TBNA performed under DS or under GA.
MATERIALS AND METHODS
Data analysis was done in accordance with the Declaration of Helsinki. The Institutional Review Board for Human Studies at our institution confirmed that a formal approval was not required as this retrospective analysis required neither an intervention nor irregularity of privacy or anonymity.
EBUS-TBNAs performed due to suspected malignancy at our institution between January 2013 and December 2016 were included in the analysis. Procedures were done by experienced investigators or under their direct supervision. Standard monitoring included electrocardiogram, oxygen saturation, and noninvasive blood pressure. EBUS-TBNAs were performed with a real-time ultrasound biopsy bronchoscope (BF-UC-160F; Olympus Ltd., Tokyo, Japan) and dedicated 22-gauge needles (NA-201SX; Olympus Ltd.).
Original data were retrieved from an electronic patient record system (Medico, Siemens, Germany). Only bronchoscopies with EBUS-TBNA performed under GA in the attendance of an anesthesiologist or under DS with fiberoptic intubation under maintenance of spontaneous breathing were included in the analysis.
Demographic (age and sex) and epidemiological data (cardiovascular or pulmonary comorbidities) were recorded and collected in a Microsoft Access database (Microsoft, Redmond, USA).[8]
In accordance with previous studies, the diagnostic yield of EBUS-TBNA, defined as the number of individuals in whom EBUS-TBNA provided a specific diagnosis, was determined.[4,9] Samples of lymph nodes were considered adequate when lymphocytes were present or when rendering a specific diagnosis whereas biopsies of lung masses were only considered as adequate when rendering a specific diagnosis.[4]
Furthermore, additional interventions (e.g., bronchoalveolar lavage) were recorded, and the bronchoscopy report and patient record system were searched for complications occurring during and after the procedure. Complications were categorized into adverse events (AEs) and severe adverse events (SAEs) as previously described.[8,10] Briefly, SAEs included death within 24 h after bronchoscopy, pneumothorax, major bleeding (defined as necessity for intubation or placement of a bronchus blocker), need for postinterventional ventilation, epileptic seizure, or any event leading to an intensive or intermediate care unit (IMC) admission. AEs included respiratory deteriorations resolving until the end of the procedure, hypotension, a prolonged recovery period, minor bleedings, difficulties with the sedation, for example, due to coughing, or any event judged as a complication of the procedure not fulfilling the definition of an SAE.
Statistical analysis was performed using GraphPad Prism (GraphPad Software, La Jolla, USA). Unless otherwise stated, all data are presented as mean ± standard deviation after testing for normal distribution (Kolmogorov–Smirnov test). A two-group comparison was performed using the unpaired t-test for normally distributed data or the Mann–Whitney test for nonnormally distributed data. The Fisher's exact test was used for categorical data. Statistical significance was defined as P < 0.05.
RESULTS
A total of 295 EBUS-TBNAs for the diagnostic workup of malignant lung diseases were done within the observed period. Procedures under investigator-directed sedation without fiberoptic intubation were excluded from the analysis (n = 30). Of the remaining 265 bronchoscopies, n = 160 were performed under GA and n = 105 were performed under DS with fiberoptic intubation [Figure 1].
Figure 1.
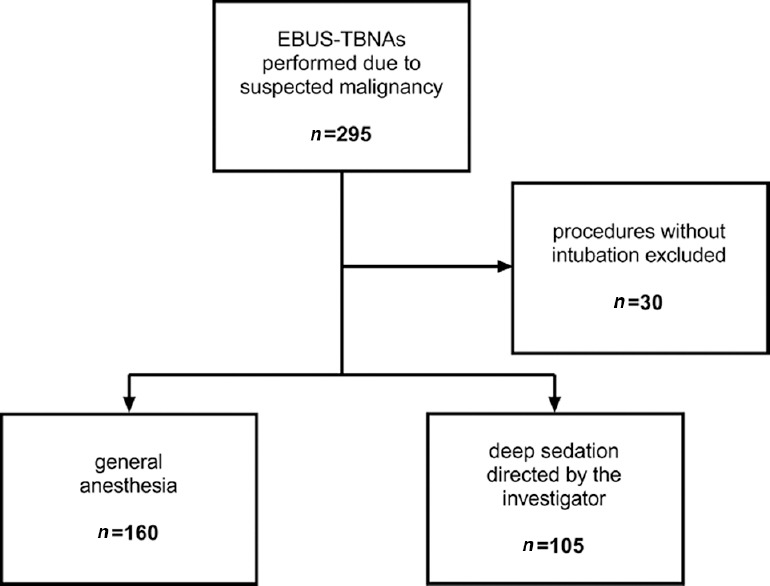
Patient flow chart
Patients in the GA and the DS group did not differ significantly in terms of sex, age, size, and the prevalence of cardiovascular or chronic pulmonary diseases [all P > 0.05; Table 1]. However, patients in the GA group were slightly heavier [Δ −4.718 ± 2.292 kg; 95% confidence interval: −9.231–−0.204 kg; P = 0.0406; Table 1].
Table 1.
Patient characteristics and diagnostic interventions
| GA (n=160) | DS (n=105) | P | |
|---|---|---|---|
| Male, n (%) | 109 (68.1) | 72 (68.6) | >0.9999* |
| Age (years) | 64.79±10.96 | 64.8±10.34 | 0.9926# |
| Weight (kg) | 79.64±18.81a | 74.93±15.42b | 0.0406# |
| Size (cm) | 172.3±9.82a | 172.5±8.41b | 0.8691# |
| Cardiovascular disease, n (%) | 108 (67.5) | 62 (59.0) | 0.1905* |
| Chronic pulmonary disease, n (%) | 70 (43.8) | 42 (40.0) | 0.6114* |
| Number of diagnostic interventions per procedure | 0.88±0.76 | 0.79±0.73 | 0.3191§ |
| Bronchoalveolar lavage, n (%) | 19 (11.9) | 5 (4.8) | 0.0516* |
| Endobronchial forceps biopsy, n (%) | 60 (37.5) | 46 (43.8) | 0.3089* |
| Endobronchial cryobiopsy, n (%) | 9 (5.6) | 3 (2.9) | 0.3739* |
| Transbronchial forceps biopsy, n (%) | 25 (15.6) | 20 (19.1) | 0.5055* |
| Transbronchial cryobiopsy, n (%) | 3 (1.9) | 1 (1.0) | >0.9999* |
Data are mean±SD or number of patients (%).*Fisher’s exact test, #student’s t-test, §Mann–Whitney test, aData were available for 152 out of 160 patiens, bData were available for 96 out of 105 patients. SD: Standard deviation, GA: General anesthesia, DS: Deep sedation
The number of diagnostic interventions apart from EBUS-TBNA was not different between the two groups [P = 0.3191; Table 1], and there were no significant differences in the proportion of procedures with bronchoalveolar lavage, endobronchial biopsies, or transbronchial biopsies [all P > 0.05; Table 1].
The number of sampled targets (lymph nodes or targets) per patient (P = 0.6693) and the number of adequate samples (P = 0.0681) did not differ significantly between the groups. There was a tendency toward a higher diagnostic yield in the DS compared to the GA group (GA: 42.5% vs. DS: 53.3%; P= 0.1018). When only patients with a final diagnosis of malignancy were used to calculate diagnostic yield, there was no significant difference between the DS and the GA group (GA: 53.6% vs. DS: 61.5%; P= 0.2675). Details about the number of samples, diagnostic yield, and final diagnoses are summarized in Table 2.
Table 2.
Number of samples, diagnostic yield of endobronchial ultrasound-guided transbronchial needle aspiration, and final diagnosis
| GA (n=160) | DS (n=105) | P | |
|---|---|---|---|
| Number of sampled LN/masses | 267 | 170 | N/A |
| Targets sampled per procedure | 1.67±0.88 | 1.62±0.81 | 0.6693# |
| Adequate samples, n (%) | 238 (89.1) | 160 (94.1) | 0.0861* |
| Diagnostic yield (all patients) (%) | 42.5 | 53.3 | 0.1018* |
| Diagnostic yield (patients with a final diagnosis of malignancy) (%) | 53.6 | 61.5 | 0.2675* |
| Diagnoses made by EBUS-TBNA, n (%) | |||
| NSCLC: Adenocarcinoma | 27 (16.9) | 23 (21.9) | N/A |
| NSCLC: SCC | 6 (3.8) | 7 (6.7) | N/A |
| NSCLC: Other | 4 (2.5) | 7 (6.7) | N/A |
| Small cell lung cancer | 18 (11.3) | 13 (12.4) | N/A |
| Other | 12 (7.5) | 6 (5.7) | N/A |
| Final diagnosis of patients with negative EBUS-TBNA, n (%) | |||
| Lung cancer | 48 (30.0) | 29 (27.6) | N/A |
| Other malignancies | 10 (6.3) | 6 (5.7) | N/A |
| No diagnosis of malignancy | 24 (15.0) | 12 (11.4) | N/A |
| No follow-up available | 11 (6.9) | 2 (1.9) | N/A |
Data are mean±SD, number of patients (%) or %. *Fisher’s exact test, #Mann–Whitney test. GA: General anesthesia, DS: Deep sedation, LN: Lymph nodes, NSCLC: Nonsmall cell lung cancer, SCC: Squamous cell carcinoma, SCLC: Small cell lung cancer, SD: Standard deviation, N/A: Not available, EBUS-TBNA: Endobronchial ultrasound-guided transbronchial needle aspiration
Details of AEs and SAEs are listed in Table 3. The proportion of interventions with AEs in the GA group was lower compared to the DS group (GA: 27.5% vs. DS: 59.1%; P < 0.0001). This was due to a higher occurrence of sedation-related problems (GA: 0% vs. DS: 28.5%; P < 0.0001) and transient respiratory deteriorations (GA: 3.8% vs. DS: 13.3%; P = 0.0153) in the DS group. Minor bleedings were common in both groups and not significantly different (P = 0.5471). In contrast, the occurrence of SAEs tended to be higher in the GA compared to the DS group (7.5% vs. 1.9% [P = 0.0523]) driven by more postinterventional intensive care unit/IMC admissions. However, the occurrence of SAEs was low in both groups.
Table 3.
Complications
| GA (n=160) | DS (n=105) | P | |
|---|---|---|---|
| AEs | 44 (27.5) | 62 (59.0) | <0.0001* |
| Difficult to sedate | 0 (0.0) | 30 (28.6) | <0.0001* |
| Minor bleedings | 33 (20.6) | 25 (23.8) | 0.5471* |
| Transient respiratory deterioration | 6 (3.8) | 14 (13.3) | 0.0073* |
| Short time mechanical ventilation | 2 (1.3) | 1 (1.0) | >0.9999* |
| Hypotension | 0 (0.0) | 2 (1.9) | 0.1561* |
| Prolonged recovery period | 0 (0.0) | 1 (1.0) | 0.3962* |
| Interruption of the procedure | 1 (0.6) | 0 (0.0) | >0.9999* |
| Other | 11 (6.9) | 10 (9.5) | 0.4889* |
| SAEs | 12 (7.5) | 2 (1.9) | 0.0523* |
| Pneumothorax | 4 (2.5) | 2 (1.9) | >0.9999* |
| ICU/IMC admission | 9 (5.6) | 0 (0.0) | 0.0128* |
| Postinterventional mechanical ventilation | 3 (1.9) | 0 (0.0) | 0.2796* |
Data are number of patients (%). *Fisher’s exact test. GA: General anesthesia, DS: Deep sedation, ICU: Intensive care unit, IMC: Intermediate care unit, SAE: Severe adverse event, AE: Adverse event
DISCUSSION
This study compared diagnostic yield and the occurrence of complications of EBUS-TBNA performed under GA or under investigator-directed DS including fiberoptic intubation. A similar diagnostic yield was observed in both groups. Several studies have analyzed the diagnostic yield of EBUS-TBNA under MS or GA with conflicting results. While data from an earlier report suggested no difference between the two approaches, a retrospective analysis done by Yarmus et al. demonstrated higher diagnostic yield when the procedure was done under DS in the presence of an anesthesiologist.[3,11] In contrast, in a recent randomized controlled trial, the diagnostic yield of EBUS-TBNA under GA was not superior compared to MS.[4] Interestingly, all these studies compared MS with GA or DS done by anesthesiologists whereas to the best of our knowledge, no data about DS directed by the bronchoscopist are available so far.
Although it has been speculated previously that the use of GA allows the sampling of more lymph nodes, we did not find differences in the number of sampled targets per procedure which is in accordance with the randomized trial done by Casal et al. and in contrast to the analysis done by Yarmus et al.[3,4]
Overall, the occurrence of AEs was significantly higher in the DS compared to the GA group, exclusively due to sedation-related problems and respiratory deteriorations. All these complications resolved by the end of the procedure and escalation of care were not necessary. Very similar observations have been made by Casal et al.[4] In contrast, admission to an intensive care unit or IMC after the procedure was required more often in the GA group in accordance with results from a large prospective registry.[2]
This study has several limitations which must be addressed. First, we had to rely on patient records to determine the occurrence of AEs and SAEs. Hence, the complication rate might have been underreported in our study. For the same reason, data about patient comfort or operating conditions during the procedure are lacking. In addition, histological confirmation or radiographic follow-up was not available for all patients with negative EBUS-TBNA which could have also led to bias. Finally, in this single-center study, EBUS-TBNA was mainly performed by experienced investigators or under their direct supervisions. Consecutively, investigator-directed DS might not necessarily be equal to GA in a different setting.
CONCLUSION
Diagnostic yield and safety of EBUS-TBNA performed under bronchoscopist-directed DS were similar compared to EBUS-TBNA performed under GA. Hence, in an appropriate setting, this approach can be used as an alternative, for example, when GA is not available.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Ernst A, Anantham D, Eberhardt R, et al. Diagnosis of mediastinal adenopathy-real-time endobronchial ultrasound guided needle aspiration versus mediastinoscopy. J Thorac Oncol. 2008;3:577–82. doi: 10.1097/JTO.0b013e3181753b5e. [DOI] [PubMed] [Google Scholar]
- 2.Eapen GA, Shah AM, Lei X, et al. Complications, consequences, and practice patterns of endobronchial ultrasound-guided transbronchial needle aspiration: Results of the AQuIRE registry. Chest. 2013;143:1044–53. doi: 10.1378/chest.12-0350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yarmus LB, Akulian JA, Gilbert C, et al. Comparison of moderate versus deep sedation for endobronchial ultrasound transbronchial needle aspiration. Ann Am Thorac Soc. 2013;10:121–6. doi: 10.1513/AnnalsATS.201209-074OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Casal RF, Lazarus DR, Kuhl K, et al. Randomized trial of endobronchial ultrasound-guided transbronchial needle aspiration under general anesthesia versus moderate sedation. Am J Respir Crit Care Med. 2015;191:796–803. doi: 10.1164/rccm.201409-1615OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Babiak A, Hetzel J, Krishna G, et al. Transbronchial cryobiopsy: A new tool for lung biopsies. Respiration. 2009;78:203–8. doi: 10.1159/000203987. [DOI] [PubMed] [Google Scholar]
- 6.Hetzel J, Eberhardt R, Herth FJ, et al. Cryobiopsy increases the diagnostic yield of endobronchial biopsy: A multicentre trial. Eur Respir J. 2012;39:685–90. doi: 10.1183/09031936.00033011. [DOI] [PubMed] [Google Scholar]
- 7.Dreher M, Cornelissen CG, Reddemann MA, et al. Nebulized versus standard local application of lidocaine during flexible bronchoscopy: A randomized controlled trial. Respiration. 2016;92:266–73. doi: 10.1159/000449135. [DOI] [PubMed] [Google Scholar]
- 8.Müller T, Thümmel K, Cornelissen CG, et al. Analogosedation during flexible bronchoscopy using a combination of midazolam, propofol and fentanyl – A retrospective analysis. PLoS One. 2017;12:e0175394. doi: 10.1371/journal.pone.0175394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aswanetmanee P, Limsuwat C, Kabach M, et al. The role of sedation in endobronchial ultrasound-guided transbronchial needle aspiration: Systematic review. Endosc Ultrasound. 2016;5:300–6. doi: 10.4103/2303-9027.191608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grendelmeier P, Tamm M, Pflimlin E, et al. Propofol sedation for flexible bronchoscopy: A randomised, noninferiority trial. Eur Respir J. 2014;43:591–601. doi: 10.1183/09031936.00200412. [DOI] [PubMed] [Google Scholar]
- 11.Herth FJ, Eberhardt R, Vilmann P, et al. Real-time endobronchial ultrasound guided transbronchial needle aspiration for sampling mediastinal lymph nodes. Thorax. 2006;61:795–8. doi: 10.1136/thx.2005.047829. [DOI] [PMC free article] [PubMed] [Google Scholar]


