Fig. 12.1.
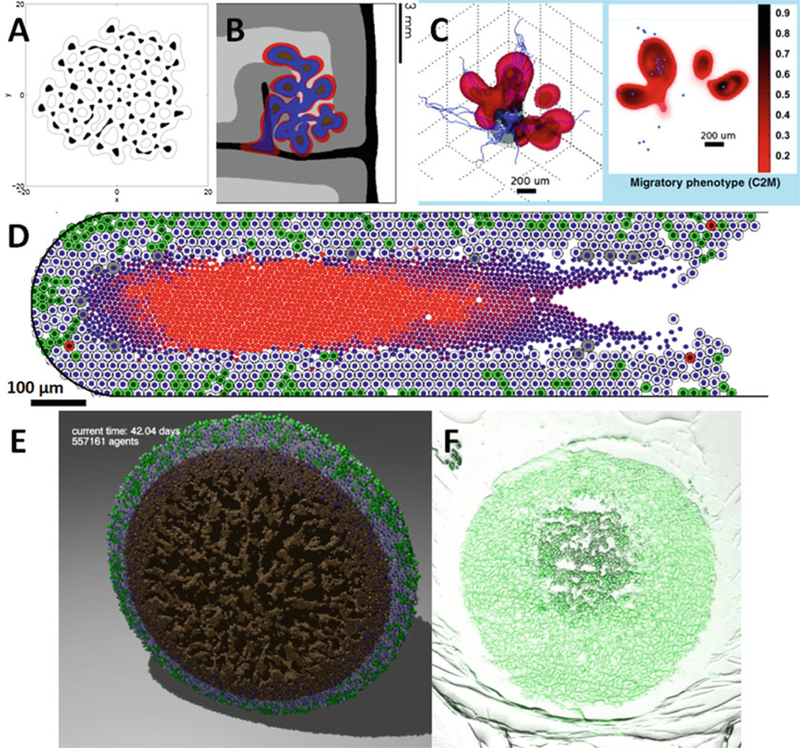
(a) A level set simulation of a tumor with viable regions (white) and necrotic tissue (black). The tumor shape can undergo complex topological changes, based upon the balance of growth and mechanics parameters (Adapted with permission from [11]). (b) Numerical refinements allowed simulation of growth in heterogeneous tissues, such as this simulated brain tumor. Red regions are proliferating, blue regions are hypoxic, and brown are necrotic. The brain tissue has white matter (light grey), grey matter (dark grey), cerebrospinal fluid (black), and cranium (white) (Adapted with permission from [52]). (c) A phase field simulation of a highly-motile subclone (red) emerging due to hypoxic signaling from a glioblastoma (grey) [7] (Adapted with permission from [7]). (d) Agent-based models—like this patient-calibrated simulation of ductal carcinoma in situ (DCIS) [19]—can simulate small-scale tissue mechanics, with more direct calibration to experimental and clinical data (Adapted with permission from [19]). (e) The agent-based model has been extended to 3D [29]. Here, we plot a cut-away view of a necrotic tumor spheroid. Green cells are proliferating, gray cells are quiescent, red cells are apoptotic, and brown cells are necrotic. Note the “crackly” structure in the necrotic core. (f) A hanging tumor drop spheroid (HCC827 non-small cell lung carcinoma) showing a similar structure in the necrotic center. Image courtesy Mumenthaler lab, Lawrence J. Ellison Institute for Transformative Medicine, University of Southern California
