Abstract
Disruption of microvascular blood flow is a common cause of tissue hypoxia in disease, yet no therapies are available that directly target the microvasculature to improve tissue oxygenation. Red blood cells (RBCs) autoregulate blood flow through S-nitroso-hemoglobin (SNO-Hb)-mediated export of nitric oxide (NO) bioactivity. We therefore tested the idea that pharmacological enhancement of RBCs using the S-nitrosylating agent ethyl nitrite (ENO) may provide a novel approach to improve tissue oxygenation. Serial ENO dosing was carried out in sheep (1–400 ppm) and humans (1–100 ppm) at normoxia and at reduced fraction of inspired oxygen (FiO2). ENO increased RBC SNO-Hb levels, corrected hypoxia-induced deficits in tissue oxygenation, and improved measures of oxygen utilization in both species. No adverse effects or safety concerns were identified. Inasmuch as impaired oxygenation is a major cause of morbidity and mortality, ENO may have widespread therapeutic utility, providing a first-in-class agent targeting the microvasculature.
Diseases of the heart, lungs, and vasculature commonly exhibit dysfunction at the microvascular level that results in deficits in tissue oxygenation despite normal oxygen levels in the blood. Currently, there are no medicines that directly target the microvasculature; thus, there are limited options to correct tissue hypoxia.
In addition to carrying oxygen, red blood cells (RBCs) autoregulate microvascular blood flow through the export of nitric oxide (NO) bioactivity. Like oxygen, NO binds avidly to heme irons in hemoglobin (Hb),1,2 but is present in low concentrations (μM for NO vs. mM for oxygen). Heme-bound NO (HbFeNO) is biologically inert, but can be activated in the lungs through a conformational change in Hb (from T to R state) that moves NO to adjacent Cysb93 thiols to form S-nitrosothiols (SNO-Hb).3,4 In hypoxic tissues, deoxygenation of Hb (inducing its transition from R to T state) promotes the release from RBCs of SNO-based vasodilatory activity. Alternatively stated, decreases in Hb oxygen saturation are coupled to conformational changes in Hb that offload bioactive NO, resulting in vasodilation. Thus, SNO-Hb increases blood flow in proportion to oxygen demand to optimize tissue oxygenation.5 The centrality of SNO-Hb in the respiratory cycle is powerfully demonstrated in mice lacking Cysb93, which exhibit profound impairments of microvascular blood flow that result in tissue hypoxia, both at baseline and in response to hypoxic insult.6,7
Vasodilation under hypoxia may be carried out by a number of different mediators, which subserve diverse pathophysiological roles; context is therefore key. Hypoxic vasodilation by SNO-Hb helps explain how RBCs link oxygen delivery with local metabolic demand8 and thus rationalizes Guyton’s classic observation that local blood flow is directly proportionate to Hb desaturation.9 This novel conceptualization of the respiratory cycle as a 3-gas system (O2/NO/CO2)3,5,6 also provides the basis for understanding why therapeutic efforts to increase the oxygen content of blood (e.g., transfusion or supplemental oxygen) can fail to improve oxygen delivery to tissues:10 that is, blood flow not blood oxygen content, is the primary determinant of oxygen delivery under physiological conditions.2,5 Consistent with this understanding of RBC physiology, decreased levels and/or impaired bioavailability of SNO-Hb have been observed in a variety of disease states characterized by tissue hypoxemia.11–16
It is important to appreciate that NO itself is inactive in RBCs and cannot effectively increase the levels of SNO-Hb.2,17 This is because NO is rapidly eliminated by oxygenated hemes in Hb, which convert it to inactive nitrate. By contrast, the conversion of NO into SNOs, which retain activity in RBCs, occurs only within a small micropopulation of Hb (i.e., only at very low NO concentrations).1,2, As a result, drugs like inhaled NO are very ineffective at replenishing SNO-Hb.17,18 We have therefore focused on alternative prodrugs that react with thiols directly to produce SNOs. Advantages of SNOs in this context include their resistance to inactivation by hemes in Hb as well as their ability to dilate microcirculatory vessels that are refractory to NO itself.19 This can be understood by appreciating that the role of guanylate cyclase (sGC)/cGMP in vasodilation by NO/SNOs varies with vessel size, and while NO is always reliant on GC to induce vasodilation, SNOs, including SNO-Hb20,21 may vasodilate by multiple mechanisms.22 Moreover, autoregulation of blood flow governing tissue oxygenation is evidently sGC-independent, as GC deficiency or inhibition has no reported effect on tissue oxygenation,23,24 in stark contrast to deficiencies in SNO-Hb.6,7 Our targeted efforts to improve microvascular blood flow controlling tissue oxygenation are thus directed at enhancing SNO-based activity.
We have developed a class of S-nitrosylating agents suitable for clinical use and have advanced a lead candidate, ethyl nitrite (ENO). Unlike inhaled NO, ENO neither liberates NO25 nor undergoes inactivation by hemes of Hb, but rather targets Cys thiols in Hb to generate SNO-Hb.25 Thus, ENO has potent vasodilatory effects mediated by RBCs,11,26–31 which may potentially correct tissue hypoxia. Here we conducted in-depth dose-ranging physiologic assessments of ENO, first in sheep (which, like humans, have a strictly conserved Cys residue at position 93 of the bchain32,33), then in healthy human subjects, under conditions of reduced oxygen availability that induce SNO-Hb-dependent vasodilation.
RESULTS
ENO dose-ranging in hypoxic sheep
A sheep model was developed to assess ENO’s pharmacologic actions on hemodynamics and arterial blood gases under hypoxia, and to determine the boundary conditions for human testing. Hemodynamic monitoring was conducted with a Swan-Ganz catheter. The sheep’s head was inserted into a gas exposure hood and, following acclimation under normoxic conditions, the fraction of inspired oxygen (FiO2) was reduced from 0.21 to 0.10. After 60 min of hypoxia, ENO was introduced into the ventilator circuit in escalating doses (1–400 ppm); each dose was administered for 45 min followed by 15 min without ENO (washout phase). (See Supplemental Methods for additional details.)
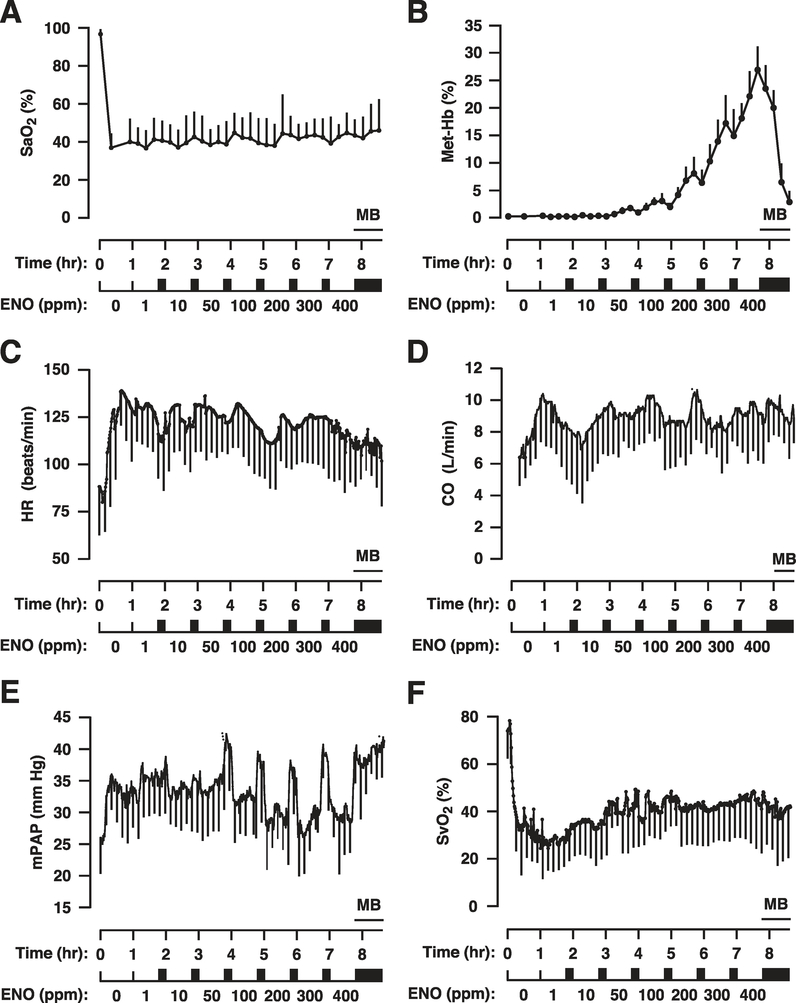
Induction of hypoxia (lowering of the FiO2 from 0.21 to 0.10) was confirmed by the rapid decline in arterial blood oxygen saturation (SaO2; 96.7 ± 2.6 to 35.6 ± 8.4%; n = 9); blood sampling (q 15 min) verified that the mean SaO2 remained between 35 and 45% for the duration of the study (Figure 1a). Deoxygenation of SNO-Hb generates metHb concomitant with release of NO bioactivity.2,34 Thus, metHb production by ENO can be used as a biomarker for SNO-Hb activity (Supplemental Figure S1). MetHb rose with increasing ENO dose: levels were well below 5% up to 100 ppm ENO, exceeded 5% at 200 ppm, and reached clinically significant levels (i.e., >10%) at >300 ppm. Importantly, metHb declined rapidly during washout, supporting its use as a biomarker. At study completion (min 510), methylene blue (1 mg/kg i.v.) was used to reduce metHb, thereby demonstrating its potential utility to correct unforeseen methemoglobinemia.
Figure 1.
Responses to ethyl nitrite (ENO) dosing during hypoxia in sheep (n = 9). Time courses are presented as mean ± standard deviation of (a) percent arterial blood oxygen saturation, SaO2%; (b) percent methemoglobin, Met-Hb%; (c) heart rate, HR, in beats per min; (d) cardiac output, CO, in liters per min; (e) mean pulmonary arterial pressure, mPAP, in mm Hg; and (f) percent systemic venous oxygen saturation, SvO2%. The standard deviations (error bars) are marked every 15 min for clarity. Hypoxia was initiated for 60 min starting at time 0 (normoxic baseline) followed by ENO dosing from 1–400 ppm with each dose administered for 45 min followed by a 15-min washout period (solid rectangles). Met-Hb levels rose with dose escalation, providing a biomarker of ENO exposure. MB demarcates the i.v. administration of 1 mg/kg methylene blue to rapidly reduce metHb levels.
Lowering FiO2 produced rapid increases in heart rate (HR), cardiac output (CO), and mean pulmonary arterial pressure (mPAP), and a decline in mixed venous oxygen saturation (SvO2; Figure 1c–f). ENO did not induce further changes in HR, SvO2, CO, or mPAP. However, during the washout periods between escalating doses of ENO, acute increases in pulmonary pressures were observed, suggesting that ENO had a PAP-lowering effect to counteract hypoxic pulmonary vasoconstriction (Figure 1e). (Rebound pulmonary hypertension cannot be excluded but has not been observed during longer-term therapy in previous studies.35) Based on the above results, the 1 and 50 ppm doses were selected for more complete testing.
Prolonged ENO exposure safety study in hypoxic sheep
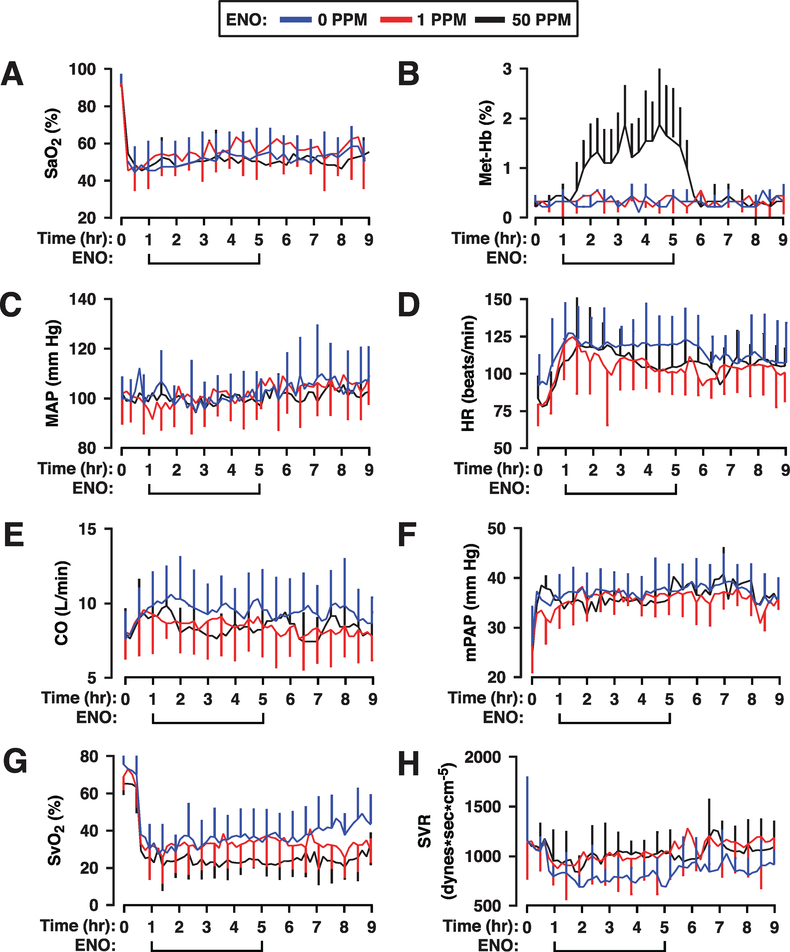
The effects of 4 h of fixed-dose exposures to ENO were tested against a hypoxic control group. A cohort of 23 sheep was distributed in three experimental groups: hypoxia control (n = 8), 1 ppm ENO treatment group (n = 8), and 50 ppm ENO treatment group (n = 7). Monitoring included both a Swan-Ganz catheter and a femoral arterial line. Following a normoxic acclimation period, the FiO2 was reduced from 0.21 to 0.10. The resultant reduction in SaO2 was similar between the three groups and was maintained over the 9-h study period (Figure 2a). Small reversible increases in metHb (1.2% to 1.8%) were seen with 50 ppm but not with 1 ppm ENO (Figure 2b).
Figure 2.
Responses during and after ethyl nitrite (ENO) dosing in hypoxic sheep. Time courses are presented as mean ± standard deviation of (a) percent arterial blood oxygen saturation, SaO2%; (b) percent methemoglobin, Met-Hb%; (c) mean arterial pressure, MAP, in mm Hg; (d) heart rate, HR, in beats per min; (e) cardiac output, CO, in liters per min; (f) mean pulmonary arterial pressure, mPAP, in mm Hg; (g) percent systemic venous oxygen saturation, SvO2%; and (h) systemic vascular resistance, SVR, in dynes*sec*cm−5. The standard deviations (error bars) are marked every 30 min for clarity. Hypoxia was initiated for 1 h starting at time 0 (normoxic baseline) then sheep received either 0 (n = 8; blue line), 1 (n = 8; red line), or 50 (n = 7; black line) ppm ENO for 4 h followed by an additional 4 h under hypoxia alone. ENO produced significant dose-dependent increases in CO and declines in SvO2 and SVR that carried into the postexposure period. See text for additional details.
The hemodynamic responses to 60 min of hypoxia were comparable between groups (Figure 2c–f) with increases recorded in CO, HR, and mPAP, while mean arterial pressure (MAP) and central venous pressure (CVP; not shown) were unchanged. Notably, hypoxia resulted in a large decrease in systemic vascular resistance (SVR) that was associated with a decline in SvO2 (Figure 2g), indicative of hypoxic vasodilation. Area-under-the-curve (AUC) comparisons were used to assess ENO-mediated changes during the 4-h treatment period. MAP (P=0.577 and 0.472; Figure 2c) and mPAP (P=0.479 and 0.386; Figure 2f) remained unchanged by either dose of ENO, indicating maintained hemodynamic stability (and no postexposure rebound pulmonary hypertension). However, both HR (1ppm, P=0.041; 50 ppm, P=0.166; Figure 2d) and CO (1ppm, P=0.059; 50 ppm, P=0.027; Figure 2e) declined with ENO, while SVR increased (P=0.053 for 1 ppm ENO and P=0.007 for 50 ppm; Figure 2h). Moreover, SvO2 declined with 50 ppm ENO (P=0.053, while remaining constant at the 1 ppm dose, P=0.479; Figure 2g), indicative of increases in peripheral oxygen extraction. Thus, ENO-associated changes in hemodynamics appear to be driven by improved tissue oxygenation.
During the hypoxic, post-ENO phase (4h), HR was unchanged in the ENO groups, while the differences with the control group diminished (P=0.166 and 0.281 for the 1 and 50 ppm groups, respectively), reflecting decreases in hypoxia-induced tachycardia in the untreated animals (Figure 2d). The drug-induced changes in SVR persisted through the postexposure period (P=0.041 and P=0.149; Figure 2h), likely reflecting the persistently low SvO2 (P=0.053 at 50 ppm; Figure 2g). Taken together, these data suggest that ENO mediates prolonged improvements in tissue oxygen extraction under hypoxia, which underlie hemodynamic changes that help to maintain hemodynamic stability.
ENO dose-ranging in hypoxic humans
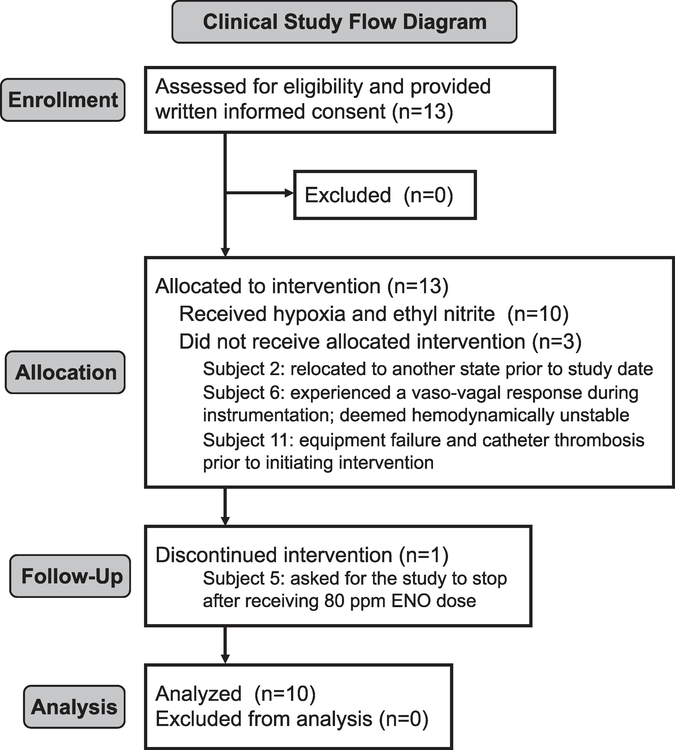
Based on the sheep findings (and a supportive toxicology study; see Supplement), we initiated a phase I safety study of ENO conducted under US Food and Drug Administration (FDA) IND 112707. Thirteen young healthy adult volunteers were recruited and 10 completed the study (Figure 3); the three withdrawals occurred prior to study initiation. Inclusion/exclusion criteria and stopping rules, along with subject demographic information, are provided in the Supplementary Materials. Following right-heart catheterization for invasive hemodynamic monitoring, each subject was fitted with an on-demand nonrebreather mask to regulate oxygen and ENO delivery; near infrared spectroscopy (NIRS) probes were placed on the forehead and calf to track changes in cerebral and skeletal muscle tissue oxygenation (StO2). Hypoxia was induced by lowering the FiO2 to 0.12. ENO was mixed into the ventilation circuit using a custom-made gas-blending device. One hundred ppm ENO was selected as the maximum dose based on the amounts of metHb observed in sheep. As an a priori safety measure, the clinical research team was instructed to terminate ENO exposure if the metHb concentration increased above 5%. Subject demographic information along with a complete list of the adverse events and corrective actions are provided in the Supplement.
Figure 3.
Human trial flow chart. Clinical trial process for subjects who volunteered to participate in the ethyl nitrite (ENO) dosing study.
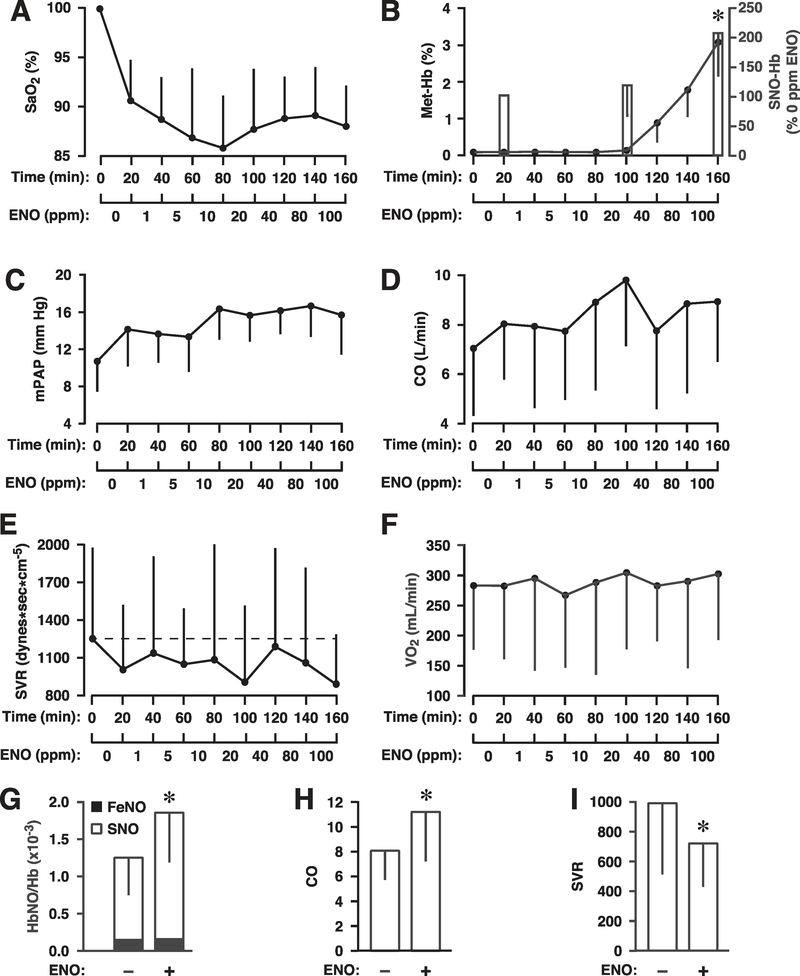
The time courses of study variables are presented in Figure 4. Lowering the FiO2 produced the expected group decline in arterial blood oxygenation (from ~100% to 90%; Figure 4a). SaO2 ranged between 86% and 90% during ENO exposure. Additional blood gas measures remained stable during the study (Supplemental Table S4). ENO did not affect blood coagulation (prothrombin time was 10.8 ± 0.6 sec at baseline and 11.4 ± 0.5 sec post 100 ppm ENO; international normalized ratio was 1.0 ± 0.1 and 1.1 ± 0.1 before and after dosing, respectively) and blood alcohol levels were always below detection (<10mg/dl after the 100 ppm dose). MetHb was undetectable below 20 ppm ENO and peaked at 3.0 ± 1.0% following the 100 ppm dose (Figure 4b). No subject required intervention to correct methemoglobinemia. The levels of SNO-Hb tracked those of metHb with no increase in iron-nitroysylHb (Figure 4g), confirming the utility of ENO as a specific S-nitrosylating agent and of metHb (generated by ENO) as a surrogate for SNO-Hb and thus a biomarker of ENO bioactivity. SNO-Hb increased modestly at the 20 ppm ENO and by over 200% at the end of the 100 ppm dose (Figure 4b; P=0.007).
Figure 4.
Responses to ethyl nitrite (ENO) during hypoxia in humans (n = 10). Time courses are presented as mean ± standard deviation of (a) percent arterial blood oxygen saturation, SaO2%; (b) percent change in S-nitrosylated hemoglobin, SNO-Hb (bars) and percent methemoglobin, Met-Hb% (line); (c) mean pulmonary arterial pressure, mPAP, in mm Hg; (d) cardiac output, CO, in liters per min; (e) systemic vascular resistance, SVR, in dynes*sec*cm−5; and (f) oxygen consumption, VO2, expressed as milliliters per min. Hypoxia was initiated at time 0 (normoxic baseline) followed by ENO dosing from 1–100 ppm with each dose administered for 20 min (no washout). Levels of SNO-Hb and Met-Hb escalated with ENO dose. Absolute values for the change in SNO-Hb, FeNOHb, and total NOHb are presented in (g) and demonstrate the selective S-nitrosylating activity of ENO. Total NOHb increased significantly from 1.27 ± 0.48 per Hb×10−3 to 1.87 ± 0.66 (P=0.005) after the 100 ppm dose, reflecting an increase in SNO-Hb (white bar; P=0.007), without change in FeNOHb (black bar; P=0.737). Also along the bottom row, group means are presented for (h) maximum CO and (i) minimum SVR (independent of ENO dose; ± ENO). CO increased significantly while SVR declined vs. their respective hypoxic baseline (all P < 0.05).
MAP and HR remained largely unchanged throughout the study, ranging between 88–95 mm Hg and 72–82 bpm, respectively (not shown). MeanPAP trended upwards with hypoxia, and on ENO, but remained within the normal range (mPAP peaked at 17 mm Hg; Figure 4c). Cardiac output (and stroke volume) increased markedly on ENO, peaking at 9.5 L/min (Figure 3d). The increase in CO was reflected in a corresponding decrease in SVR, which reached a nadir of 675 dynes × sec × cm−5 (Figure 4e,i); these two parameters combined to maintain a near-constant level of oxygen consumption (VO2; Figure 4f). Mean arterial–venous (A-V) differences in oxygen content and the oxygen extraction ratio also did not significantly change during the study, confirming that tissues were regulating their own oxygen needs during global hypoxia. Thus, hemodynamic changes induced by ENO were likely mediated by a dose-dependent decline in SVR, consistent with a determinative role of hypoxic vasodilation by RBCs. In fact, the group differences in key measures of hypoxic physiology (CO, SVR, and SNO-Hb) showed that, independent of ENO dose, every subject experienced a significant rise in CO, decline in SVR, and increase in SNO-Hb levels (Figure 4g–i). Importantly, measurements of muscle oxygenation were fully supportive of this interpretation.
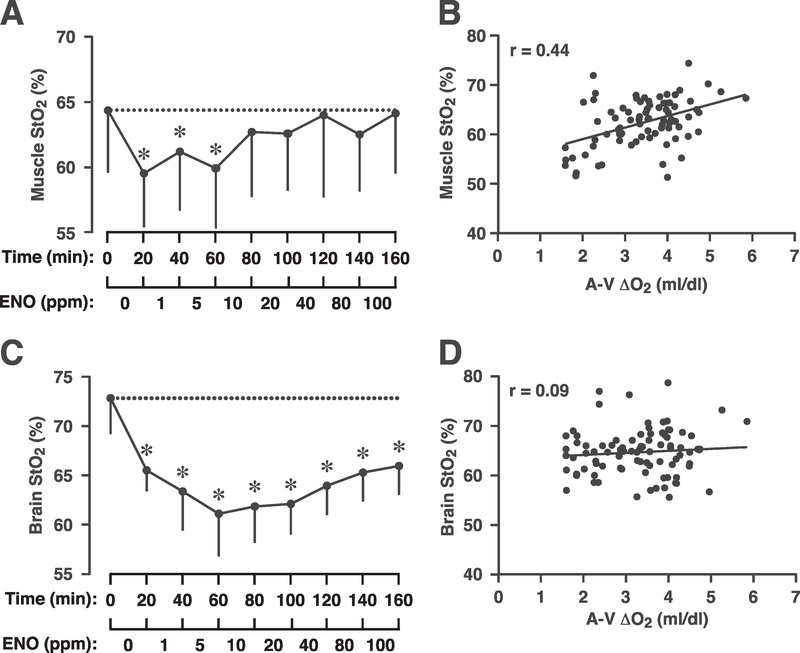
Skeletal muscle StO2 values are presented in Figure 5a. Induction of hypoxia resulted in the expected decline in muscle StO2 (P=0.01). Remarkably, however, skeletal muscle StO2 then rose significantly on ENO, such that calf oxygenation was ultimately restored to the normoxic baseline (P=1.00). Further, muscle StO2 directly correlated with A-V oxygen content differences (Figure 5b; r=0.44): as the A-V difference widened, calf oxygenation increased. Taken together, these data strongly support the interpretation that ENO improved skeletal muscle oxygen delivery and utilization. By contrast, cerebral oxygenation, which is primarily regulated by changes in carbon dioxide,36 remained at or near the hypoxic level throughout ENO dose-escalation (Figure 5c; P < 10−7) and there was no correlation between brain oxygenation and A-V difference (Figure 5d; r=0.09). (The difference in StO2 values between brain and calf reflect the dissimilar makeup of these two distinct vascular beds and not a physiological difference in oxygenation.)
Figure 5.
Effect of ethyl nitrite (ENO) on tissue oxygenation (StO2) and oxygen utilization in humans. (a) Calf muscle oxygenation measured with near infrared spectroscopy during hypoxia and dosing with 1–100 ppm ENO; each dose was administered for 20 min (no washout). Initiation of hypoxia (time 0; normoxic baseline) produced a significant decline in StO2 (*P < 0.05). For doses >10 ppm ENO, calf oxygenation increased, reaching normoxic levels at 40 ppm. At study completion, muscle StO2 was at normoxic (prehypoxic) levels (P=1.00). (b) Consistent with an ENO-induced increase in peripheral oxygen utilization there was a direct linear relationship between muscle StO2 and arterial-venous oxygen content difference (A-V Δ O2, mL/dL; r=0.44). (c) Brain StO2 declined with hypoxia, then remained significantly lower than the prehypoxic starting point throughout ENO dosing (*P < 10−7). (d) Consistent with the persistent decrease in cerebral oxygenation, there was no relationship between brain StO2 and A-V Δ O2 difference (r=0.09).
DISCUSSION
S-Nitrosohemoglobin has an essential function in oxygenating tissues that is mediated by vasodilation of the microvasculature.2,6,7 Small vessel dilation by SNO-Hb is proportional to the degree of hypoxia, which ensures that blood flow is coupled to local metabolic demand. Mechanistically, hypoxia evokes a conformational change in SNO-Hb that liberates vasoactive SNO.2 Conditions associated with low SNO-Hb are therefore characterized by aberrant blood flow and tissue hypoxia. Conversely, therapies directed towards increasing SNO-Hb may have unique potential to correct microvascular blood flow and thus treat manifold disorders of tissue oxygenation.
Here we report preliminarily on the efficacy of ENO as a first-in-class S-nitrosylating agent targeting the microvasculature. Studies were performed at both normoxia and hypoxia to evaluate the importance of the allosteric mechanism in SNO-Hb that mediates hypoxic vasodilation. (The degree of hypoxia was designed to produce clinically relevant resting SaO2 levels seen in patients with oxygenation pathologies.) Taken together with previous studies, 11,28,29,35,37 which have established the safety of ENO in hypoxic patients, the data offer compelling support for the idea that administration of ENO can target RBC Hb to safely improve tissue oxygenation (and oxygen utilization) under conditions of reduced oxygen availability. Although the postexposure effects of ENO lasted for at least several hours in sheep, further work is needed to delineate the effect duration in humans (including how long SNO-Hb levels remain elevated). This limitation notwithstanding, we emphasize that the ability of ENO to selectively lower SVR under hypoxia while improving tissue oxygenation in muscle (effectively recapitulating hypoxic physiology) are unprecedented among therapeutic agents.
Thirty years after the identification of endothelium-derived relaxing factor as NO,38,39 or a closely related S-nitrosothiol,21 the role of NO bioactivity in the regulation of blood flow and oxygen delivery continues to be the focus of intense pharmacologic interest. This line of research is based on the premise that enhancing NO bioactivity may be a viable therapeutic goal to correct vascular pathologies. The best example is inhaled NO, which selectively lowers pulmonary pressures in association with increases in blood oxygenation.40 However, as noted in the introduction, the mechanistic effects of inhaled NO derive primarily from activation of sGC that increases cGMP levels in the lung to improve ventilation/perfusion (V/Q) matching, not through increasing microvascular blood flow in tissues. In addition, increases in peripheral blood flow through endothelial NO/sGC (e.g., L-arginine, tetrahydrobiopterin, sildenafil) are mechanistically distinct from those governing tissue oxygenation, which are eNOS-independent41 and mediated by RBCs.2 RBC-induced vasodilation is carried out by SNOs,2, which retain biological activity in the presence of Hb. Notably, SNOs are far more potent than NO itself in the microcirculation,19 likely reflecting a mechanism of vasodilation that is independent of cGMP. The latter interpretation is consistent with cGMP-independent activity of SNO-Hb42 and the finding that methylene blue did not affect ENO-mediated improvements in systemic hemodynamics (Figure 1).
The matching of flow to oxygen requirements of tissues by RBCs (referred to as blood flow autoregulation) is an integral part of the mammalian respiratory cycle.5 Vasodilation subserving autoregulation of blood flow is directly proportionate to the degree of hypoxia, which distinguishes it from other forms of vasorelaxation.42 For example, both eNOS activity and nitrite-derived NO may increase under hypoxia, but neither increases proportionately to the degree of hypoxia (nitrite-derived NO is maximal at the P50 of Hb, i.e., pO2 ~27 mm Hg, which should exclude a role in autoregulation). Further, neither inhibition of eNOS nor nitrite administration has been shown to have any effect on autoregulation of blood flow or tissue oxygenation, whereas SNO-Hb meets criteria by stringent biochemical, pharmacological, and genetic standards. First, vasodilation by SNO-Hb (and offloading of NO bioactivity more generally) is directly proportional to the degree of hypoxia across the full range of Hb oxygen saturation. Second, administration of SNO-Hb and repletion of RBC-SNOs improves tissue oxygenation. Third, a deficiency of SNO-Hb leads to profound loss of physiological autoregulation.5,11 This understanding motivates development of drugs that target Hb to generate SNO-Hb.
As we show here, and have reported previously,11 ENO selectively increases circulating SNO-Hb levels in humans with accompanying improvements in tissue oxygenation that are mediated by RBCs. However, SNO-Hb is not an ideal biomarker to track drug exposure, as current technology does not allow for point-of-care quantification. Also mechanistically, the vasodilation by SNO-Hb is not a linear function of concentration, but rather coupled to oxygen saturation of Hb.2,43 For these reasons, we monitored metHb (Figures 1b and 2b for sheep; Figure 4b for human). MetHb formation by ENO is directly correlated with ENO exposure and with vasodilatory activity. This is because ENO forms metHb via SNO-Hb (metHb is generated during NO release from SNO-Hb),2,34 rather than by reacting with oxygenated hemes like NO. Since ENO is administered in the lungs, where Hb is fully oxygenated, metHb is a useful biomarker of SNO-based activity. ENO will also react with vacant hemes in Hb under hypoxic conditions to produce nitrosyl metHb. However, since nitrosyl metHb is a precursor of SNO-Hb,1 amounts of metHb will be generally correlated with SNO, even if metHb exceeds SNO-Hb.
Our previous ENO studies were performed in infants35 and adults11 with lung diseases; the observed beneficial effects were attributed to improvements in V/Q matching. Our new data establish ENO’s effects on peripheral tissues and fully support an expanded model of the respiratory cycle that is based on the coordinated transport of three gases (O2/NO/CO2).5 In this model, the delivery of NO bioactivity conveyed through RBCs is coupled to oxygen delivery in a process governed by Hb allostery.2,43 A reduction in pO2 (hypoxia) promotes the deoxygenated conformation (T-structure) in Hb that coordinately liberates both NO and oxygen to match blood flow with regional metabolic demand.42,43 Restoring or enhancing RBC SNO-Hb levels may thus correct deficits in oxygenation.
At first glance, some of the cardiovascular responses to hypoxia vary between sheep and humans, most notably the decline in CO and rise in SVR in the 4-h fixed dose in sheep as opposed to the general rise in CO and decline in SVR in humans. Thus, it is important to recognize the differences between protocols, including duration of ENO exposure (e.g., the decline in sheep CO and increase in SVR became more apparent after 2 h) and the degree of hypoxia (FiO2 of 0.10 vs. 0.12 for sheep and humans, respectively). Nonetheless, both the sheep and human studies demonstrated consistent ENO-mediated improvements in oxygenation, suggesting that changes in hemodynamics were consequent upon and driven by changes in tissue oxygenation—an indication of physiological autoregulation. Consistent with this interpretation, ENO increased circulating SNO-Hb as well as measures of oxygenation in both humans and sheep (at constant or increasing pCO2). The response to S-nitrosylation therapy during reduced oxygen availability is thus best rationalized by restoration of microcirculatory blood flow, which is solidly supported by the calf NIRS measures and the positive relationship between muscle StO2 and A-V oxygen content difference (Figure 5a,b).
In contrast to skeletal muscle, ENO failed to produce a significant increase in human cerebral oxygenation after induction of hypoxia (Figure 5c). This reflects, perhaps, the primary role of carbon dioxide in cerebral blood flow regulation36 and the likelihood that the brain will maximize vasodilation under hypoxia,44 thus limiting the effect of increasing SNO-Hb. Interestingly, a nitrate-supplemented diet, which increases blood SNOs,45,46 has been reported to improve muscle StO2 but not central nervous system oxygenation in hypoxic humans.47 We have previously reported on ENO’s ability to improve cerebral blood flow and oxygen delivery to areas of focal hypoxia/ischemia in a preclinical model of subarachnoid hemorrhage,28 and we have demonstrated that SNO-Hb in fact mediates autoregulation in the brain of small animals.48 As a result, it is reasonable to suggest that global hypoxia may not represent a condition under which SNO-Hb plays a substantial role in cerebral oxygenation and may not be the most appropriate experimental preparation to fully characterize the brain’s response to S-nitrosylation therapy.
Microvascular dysfunction with impaired blood flow is now a recognized hallmark of many respiratory, cardiovascular, and blood disorders. Mechanistic understanding of such conditions has increasingly converged on hypoxic regulation of blood flow, which entails a central role for RBCs. In this model, RBCs carry out an essential function in oxygenating tissues that is largely independent of RBC mass (i.e., blood oxygen-carrying capacity), concordant with accumulating evidence that blood flow dominates respiratory cycle physiology. Our results confirm that therapeutic targeting of RBCs to enhance SNO-mediated activity can increase blood flow and oxygen delivery to hypoxic tissues. No currently approved therapeutics, including vasodilators, oxygen therapy, or RBC transfusion, demonstrate this therapeutic profile. Accordingly, follow-on testing of ENO in disease populations characterized by pathologies of tissue oxygenation may be worthwhile.
METHODS
Animal research
Animal studies were approved by the Institutional Animal Care and Use Committee of Duke University; Duke is an AAALAC-accredited and PHS-assured institution. Procedures, monitoring, and care of the sheep complied with the Guide for the Care and Use of Laboratory Animals.
Adult mix-breed sheep (obtained from local commercial suppliers) were used to assess ENO’s pharmacologic actions on hemodynamics and arterial blood gases under hypoxia, and to determine the boundary conditions for human testing. All potentially painful procedures were conducted under an appropriate plane of anesthesia. Blood gas status (including metHb level) was quantified using a Gem Premier 3000 clinical blood gas analyzer linked to a Gem OPL co-oximeter (Instrumentation Laboratory, Lexington, MA). At the end of the experiment, each sheep was humanely killed by an approved method listed in the AVMA Guidelines on Euthanasia. Additional descriptions of the methods used for invasive hemodynamic and noninvasive tissue oxygenation monitoring and blood assays are provided in the Supplement.
Human subjects research
Clinical studies were approved by the Institutional Review Board of University Hospitals Cleveland Medical Center (UHCMC) and conducted under FDA IND 112707; UHCMC is an AAHRPP-accredited institution and an independent data and safety monitoring board was in place to review the procedures and adjudicate any adverse events. Written informed consent was obtained from each individual prior to their voluntary participation in the study. This study was not registered, as it was a phase I trial.
All aspects of the study protocol followed good clinical practice guidelines. A CONSORT-type flow diagram is depicted in Figure 3. Inclusion/exclusion criteria and stopping rules are provided in the Supplement. Following right-heart catheterization for invasive hemodynamic monitoring, healthy volunteers were fitted with an on-demand non-rebreather mask to regulate oxygen and ENO delivery. Hypoxia was induced by lowering the FiO2 to 0.12. ENO was mixed into the ventilation circuit using a custom-made gas-blending device (Custom Gas Solutions; Durham, NC); ENO was prepared solely for our use under good manufacturing procedures by the same company. Photolysis-chemiluminescence was used to quantify RBC SNO-Hb levels before and during ENO exposure.49, 50 Blood gases (including metHb levels) were quantified using a Gem Premier 4000 clinical blood gas analyzer linked to a Gem OPL co-oximeter (Instrumentation Laboratory). Further clinical chemistry safety assessments included measurements of coagulation parameters and blood alcohol concentration (ethanol is produced as a byproduct of ENO’s reaction with thiols). Additional details can be found in the Supplement.
Statistical analysis
For continuously recorded data, median values were calculated at 1-min intervals and averaged to obtain group data. For normally distributed data, analysis of variance (ANOVA) followed by post-hoc testing was used to determine within-group differences over time and differences between the experimental groups. Differences in discrete variables were tested for using t-tests. Where appropriate, testing was conducted using the equivalent nonparametric test (Kruskal–Wallis, Mann–Whitney). Results were considered significant at P < 0.05.
In order to assess ENO-mediated changes during the 4-h treatment period, a Wilcoxon Signed Rank test was conducted. The first step was to calculate the AUC by taking the sum of all data points within each stage for each sheep. The next step was comparing the AUC for different stages by performing a two-sample Wilcoxon test on the vectors of sums.
The Wilcoxon Signed Rank test was also used to compare different ENO dosage back to normoxia for both muscle and brain saturations. The AUC for each dosage was calculated by taking the sum of all recorded data within each stage for all patients, then comparing the calculated area of each stage with the area under normoxia. Linear regression was conducted to determine the association between muscle and brain StO2 and arterial-venous blood oxygen content.
Supplementary Material
Study Highlights.
WHAT IS THE CURRENT KNOWLEDGE ON THE SUBJECT?
☑ Many heart, lung, and blood disorders are associated with microvascular pathology that impairs oxygen delivery, yet there are limited therapeutic options to correct these deficits in blood flow. Blood flow governing tissue oxygenation is controlled by S-nitrosohemoglobin (SNO-Hb) in red blood cells (RBCs). Decreased levels of SNO-Hb have been observed in disease states characterized by tissue hypoxemia. These observations raise the idea that tissue oxygenation may be improved by increasing the S-nitrosylation of Hb.
WHAT QUESTION DID THIS STUDY ADDRESS?
☑ Can tissue oxygenation be improved under hypoxic conditions by raising the SNO-Hb content of blood?
WHAT THIS STUDY ADDS TO OUR KNOWLEDGE
☑ Administration of the novel S-nitrosylating agent ethyl nitrite (ENO) under hypoxia increased RBC SNO-Hb levels, corrected hypoxia-induced deficits in tissue oxygenation, and improved measures of oxygen utilization in both sheep and humans.
HOW THIS MIGHT CHANGE CLINICAL PHARMACOLOGY OR TRANSLATIONAL SCIENCE
☑ To the extent that impaired tissue oxygenation is a major cause of human morbidity and mortality, ENO therapy could have widespread utilization as a therapeutic intervention.
ACKNOWLEDGMENTS
We thank the subjects who volunteered to participate in this study. We also acknowledge the important regulatory management of this project conducted by Ms. Jenna Stump, MS, CCRP, the nursing support provided by Ms. Marianne Vest, MA, RN, CTTS, and the clinical guidance provided to the research team by the DSMB members Drs. Sri Madan Mohan, Howard Nearman, and Kingman Strohl.
FUNDING
This work was sponsored in part by Defense Advanced Research Projects Agency contracts N66001-10-C-2015 and N66001-13-C-4054, a contract from Nivalis Therapeutics (formerly Nitrox), and grants from the Clinical and Translational Science Collaborative of Cleveland, 4UL1TR000439, funded by the National Center for Advancing Translational Sciences component of the National Institutes of Health and NIH roadmap for Medical Research. The publication’s contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Footnotes
CONFLICT OF INTEREST
Dr. Reynolds has a financial interest in Miach Medical Innovations. Dr. Stamler has financial interests in Nivalis Therapeutics (formerly Nitrox), Adamas Pharma, LifeHealth, and Vindica Pharm. Drs. Moon, Piantadosi, Reynolds, and Stamler hold patents related to renitrosylation of blood, some of which have been licensed for commercial development. Both institutions are aware of these conflicts and appropriate management plans are in place. None of the other authors have relevant conflicts to disclose.
Additional Supporting Information may be found in the online version of this article.
References
- 1.Angelo M, Singel DJ & Stamler JS An S-nitrosothiol (SNO) synthase function of hemoglobin that utilizes nitrite as a substrate. Proc. Natl. Acad. Sci. U. S. A 103, 8366–8371 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Singel DJ & Stamler JS Chemical physiology of blood flow regulation by red blood cells: the role of nitric oxide and S-nitrosohemoglobin. Annu. Rev. Physiol 67, 99–145 (2005). [DOI] [PubMed] [Google Scholar]
- 3.McMahon TJ et al. Nitric oxide in the human respiratory cycle. Nat. Med 8, 711–717 (2002). [DOI] [PubMed] [Google Scholar]
- 4.Gow AJ & Stamler JS Reactions between nitric oxide and haemoglobin under physiological conditions. Nature 391, 169–173 (1998). [DOI] [PubMed] [Google Scholar]
- 5.Doctor A & Stamler JS Nitric oxide transport in blood: a third gas in the respiratory cycle. Comprehen. Phys 1, 541–568 (2011). [DOI] [PubMed] [Google Scholar]
- 6.Zhang R et al. Hemoglobin βCys93 is essential for cardiovascular function and integrated response to hypoxia. Proc. Natl. Acad. Sci. U. S. A 112, 6425–6430 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang R, Hess DT, Reynolds JD & Stamler JS Hemoglobin S-nitrosylation plays an essential role in cardioprotection. J. Clin. Invest 126, 4654–4658 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Doctor A et al. Hemoglobin conformation couples erythrocyte S-nitrosothiol content to O2 gradients. Proc. Natl. Acad. Sci. U. S. A 102, 5709–5714 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ross JM, Fairchild HM, Weldy J & Guyton AC Autoregulation of blood flow by oxygen lack. Am. J. Physiol 202, 21–24 (1962). [DOI] [PubMed] [Google Scholar]
- 10.Hodges AN, Delaney S, Lecomte JM, Lacroix VJ & Montgomery DL Effect of hyperbaric oxygen on oxygen uptake and measurements in the blood and tissues in a normobaric environment. Br. J. Sports Med 37, 516–520 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McMahon TJ et al. A nitric oxide processing defect of red blood cells created by hypoxia: deficiency of S-nitrosohemoglobin in pulmonary hypertension. Proc. Natl. Acad. Sci. U. S. A 102, 14801–14806 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pawloski JR, Hess DT & Stamler JS Impaired vasodilation by red blood cells in sickle cell disease. Proc. Natl. Acad. Sci. U. S. A 102, 2531–2536 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.James PE, Lang D, Tufnell-Barret T, Milsom AB & Frenneaux MP Vasorelaxation by red blood cells and impairment in diabetes: Reduced nitric oxide and oxygen delivery by glycated hemoglobin. Circ. Res 94, 976–983 (2004). [DOI] [PubMed] [Google Scholar]
- 14.Crawford JH et al. Transduction of NO-bioactivity by the red blood cell in sepsis: novel mechanisms of vasodilation during acute inflammatory disease. Blood 104, 1375–1382 (2004). [DOI] [PubMed] [Google Scholar]
- 15.Liu L et al. Essential roles of S-nitrosothiols in vascular homeostasis and endotoxic shock. Cell 116, 617–628 (2004). [DOI] [PubMed] [Google Scholar]
- 16.Datta B et al. Red blood cell nitric oxide as an endocrine vasoregulator: a potential role in congestive heart failure. Circulation 109, 1339–1342 (2004). [DOI] [PubMed] [Google Scholar]
- 17.Terpolilli NA et al. Inhalation of nitric oxide prevents ischemic brain damage in experimental stroke by selective dilatation of collateral arterioles. Circ. Res 110, 727–738 (2012). [DOI] [PubMed] [Google Scholar]
- 18.McMahon TJ & Doctor A Extrapulmonary effects of inhaled nitric oxide: role of reversible S-nitrosylation of erythrocytic hemoglobin. Proc. Am. Thorac. Soc 3, 153–160 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sellke FW, Myers PR, Bates JN & Harrison DG Influence of vessel size on the sensitivity of porcine coronary microvessels to nitroglycerin. Am. J. Physiol 258, H515–520 (1990). [DOI] [PubMed] [Google Scholar]
- 20.Pawloski JR & Stamler JS Vasoactivity and SNO levels of RBCs. FASEB J. 16, A850–A850 (2002). [Google Scholar]
- 21.Lima B, Forrester MT, Hess DT & Stamler JS S-Nitrosylation in cardiovascular signaling. Circ. Res 106, 633–646 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bolotina VM, Najibi S, Palacino JJ, Pagano PJ & Cohen RA Nitric-oxide directly activates calcium-dependent potassium channels in vascular smooth-muscle. Nature 368, 850–853 (1994). [DOI] [PubMed] [Google Scholar]
- 23.Wei HM, Tse J, Chi OZ & Weiss HR Effects of topical methylene blue on cyclic GMP level, blood flow, and O2 consumption in focal cerebral ischaemia. Neurol. Res 16, 449–455 (1994). [DOI] [PubMed] [Google Scholar]
- 24.Ashkenazi I, Rubenstein I, Abassi Z, Kluger Y & Olsha O Methylene blue does not inhibit capillary blood flow increase in crush injury. J. Appl. Med. Sci 4, 75–84 (2015). [Google Scholar]
- 25.Moya MP et al. S-Nitrosothiol repletion by an inhaled gas regulates pulmonary function. Proc. Natl. Acad. Sci. U. S. A 98, 5792–5797 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Auten RL et al. Inhaled ethyl nitrite prevents hyperoxia-impaired postnatal alveolar development in newborn rats. Am. J. Respir. Crit. Care Med 176, 291–299 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reynolds JD et al. S-Nitrosylation therapy to improve oxygen delivery of banked blood. Proc. Natl. Acad. Sci. U. S. A 110,11529–11534 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sheng H et al. Pharmacologically augmented S-nitrosylated hemoglobin improves recovery from murine subarachnoid hemorrhage. Stroke 42, 471–476 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shimazutsu K et al. Inclusion of a nitric oxide congener in the insufflation gas repletes S-nitrosohemoglobin and stabilizes physiologic status during prolonged carbon dioxide pneumoperitoneum. Clin. Transl. Sci 2, 405–412 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yurcisin BM et al. Repletion of S-nitrosohemoglobin improves organ function and physiological status in swine after brain death. Ann. Surg 257, 971–977 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jia L, Bonaventura C, Bonaventura J & Stamler JS S-Nitrosohaemoglobin: a dynamic activity of blood involved in vascular control. Nature 380, 221–226 (1996). [DOI] [PubMed] [Google Scholar]
- 32.Boyer SH et al. Differences in the amino acid sequences of tryptic peptides from three sheep hemoglobin pchains. J. Biol. Chem 242, 2211–2232 (1967). [PubMed] [Google Scholar]
- 33.Nagai K, Perutz MF & Poyart C Oxygen binding-properties of human mutant hemoglobins synthesized in Escherichia coli. Proc. Natl. Acad. Sci. U. S. A 82, 7252–7255 (1985). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pezacki JP, Ship NJ & Kluger R Release of nitric oxide from S-nitrosohemoglobin. Electron transfer as a response to deoxygenation. J. Am. Chem. Soc 123, 4615–4616 (2001). [DOI] [PubMed] [Google Scholar]
- 35.Moya MP, Gow AJ, Califf RM, Goldberg RN & Stamler JS Inhaled ethyl nitrite gas for persistent pulmonary hypertension of the newborn. Lancet 360, 141–143 (2002). [DOI] [PubMed] [Google Scholar]
- 36.Paulson OB, Strandgaard S & Edvinsson L Cerebral autoregulation. Cerebrovasc. Brain Metab. Rev 2, 161–192 (1990). [PubMed] [Google Scholar]
- 37.Ali NA et al. A method to attenuate pneumoperitoneum-induced reductions in splanchnic blood flow. Ann. Surg 241, 256–261 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ignarro LJ, Buga GM, Wood KS, Byrns RE & Chaudhuri G Endothelium-derived relaxing factor produced and released from artery and vein is nitric oxide. Proc. Natl. Acad. Sci. U. S. A 84, 9265–9269 (1987). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Palmer RM, Ferrige AG & Moncada S Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature 327, 524–526 (1987). [DOI] [PubMed] [Google Scholar]
- 40.Ichinose F, Roberts JD Jr. & Zapol WM Inhaled nitric oxide: a selective pulmonary vasodilator: Current uses and therapeutic potential. Circulation 109, 3106–3111 (2004). [DOI] [PubMed] [Google Scholar]
- 41.Cabrales P, Tsai AG, Frangos JA & Intaglietta M Role of endothelial nitric oxide in microvascular oxygen delivery and consumption. Free Radic. Biol. Med 39, 1229–1237 (2005). [DOI] [PubMed] [Google Scholar]
- 42.Allen BW, Stamler JS & Piantadosi CA Hemoglobin, nitric oxide and molecular mechanisms of hypoxic vasodilation. Trends Mol. Med 15, 452–460 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McMahon TJ, Stone AE, Bonaventura J, Singel DJ & Stamler JS Functional coupling of oxygen binding and vasoactivity in S-nitrosohemoglobin. J. Biol. Chem 275, 16738–16745 (2000). [DOI] [PubMed] [Google Scholar]
- 44.Wilson MH et al. Cerebral artery dilatation maintains cerebral oxygenation at extreme altitude and in acute hypoxia—an ultrasound and MRI study. J. Cereb. Blood Flow Metab 31, 2019–2029 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pinheiro LC et al. Gastric S-nitrosothiol formation drives the antihypertensive effects of oral sodium nitrite and nitrate in a rat model of renovascular hypertension. Free Radio. Biol. Med 87, 252–262 (2015). [DOI] [PubMed] [Google Scholar]
- 46.Montenegro MF et al. Blood pressure-lowering effect of orally ingested nitrite is abolished by a proton pump inhibitor. Hypertension 69, 23–31 (2017). [DOI] [PubMed] [Google Scholar]
- 47.Masschelein E et al. Dietary nitrate improves muscle but not cerebral oxygenation status during exercise in hypoxia. J. Appl. Physiol. (1985). 113, 736–745 (2012). [DOI] [PubMed] [Google Scholar]
- 48.Stamler JS et al. Blood flow regulation by S-nitrosohemoglobin in the physiological oxygen gradient. Science 276, 2034–2037 (1997). [DOI] [PubMed] [Google Scholar]
- 49.McMahon TJ & Stamler JS Concerted nitric oxide/oxygen delivery by hemoglobin. Methods Enzymol. 301, 99–114 (1999). [DOI] [PubMed] [Google Scholar]
- 50.Hausladen A et al. Assessment of nitric oxide signals by triiodide chemiluminescence. Proc. Natl. Acad. Sci. U. S. A 104, 2157–2162 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.