Figure 1.
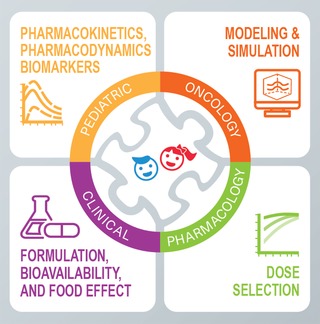
Role of clinical pharmacology in pediatric oncology drug development. Strategically designed PK/PD and biomarker sampling in pediatric oncology trials enables collection of the right data, which feed into advanced and prespecified modeling and simulation approaches, thus enabling rational dose selection for modern molecularly targeted cancer therapies in children. Extrapolation of relative bioavailability information to inform dosing using physiologically based PK modeling enables acceleration of pediatric oncology drug development when studies in adults are limited or absent. Clinical pharmacology tools represent an essential piece of the challenging pediatric oncology drug development puzzle. PD, pharmacodynamics; PK, pharmacokinetics.
