Figure 2.
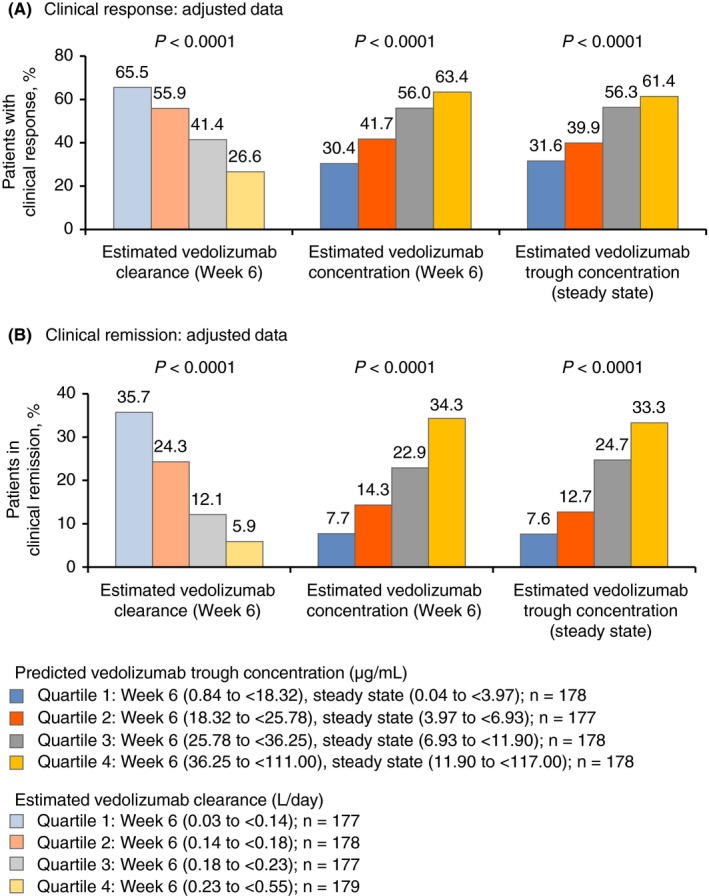
Clinical response (A) and remission (B) (adjusted for age, weight, history of prior anti‐TNFα therapy, serum albumin concentration and faecal calprotectin concentration), stratified by quartiles from low (quartile 1) to high (quartile 4) for estimated concentration and estimated clearance of vedolizumab at week 6 and steady state (n = 170‐177). P values were determined using the exact Cochran‐Armitage trend test. Clinical response was defined as a reduction in partial Mayo Score of ≥3 points and a ≥30% decrease from baseline, with a decrease of ≥1 point on the rectal bleeding sub‐score or an absolute rectal bleeding score ≤1. Clinical remission was defined as a partial Mayo Score of ≤2 points with no individual sub‐score >1 point and mucosal healing (endoscopic sub‐score ≤1). TNFα, tumour necrosis factor alpha
