Figure 2.
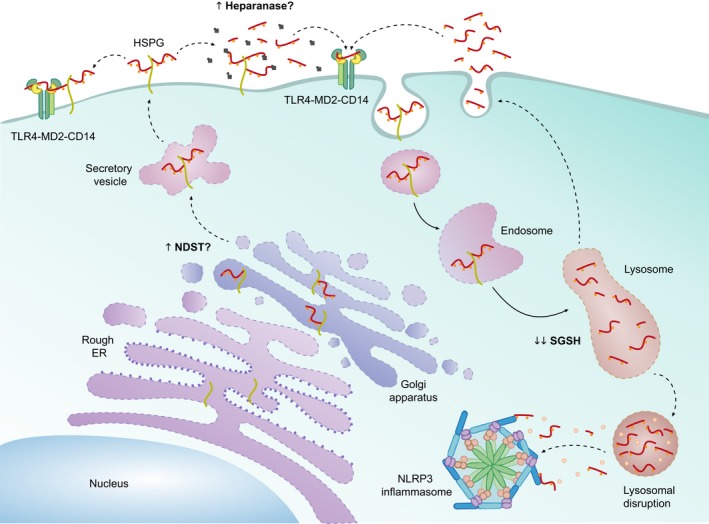
Proposed role of MPSIIIA heparan sulphate (HS) in innate immunity. HS is synthesised in the endoplasmic reticulum‐Golgi, where it undergoes chain modification events. It is likely that MPSIIIA HS is highly sulphated due to an increase in activity of the chain modification enzyme NDST. HS bound to a proteoglycan (HSPG) is exocytosed to the cell membrane. In its HSPG form it may interact with the TLR4‐MD2‐CD14 complex to propagate an inflammatory response. HS may also be cleaved by heparinase, an endoglycosidase which has been seen to be up‐regulated in cancer metastasis and neuroinflammation. Increased heparanase activity would digest HS to short fragments which may have the potential to bind the TLR4‐MD2‐CD14 complex. In order to degrade HS, HSPGs are endocytosed and digested within the endolysosome, the deficiency in SGSH which is the primary cause of MPSIIIA, would result in HS fragments which are highly sulphated with a sulphated non‐reducing end residue. These fragments may either undergo exocytosis and subsequent binding to the TLR4 complex, or be released intracellularly due to lysosomal destabilisation and directly activate the NLRP3 inflammasome.
