Figure 1.
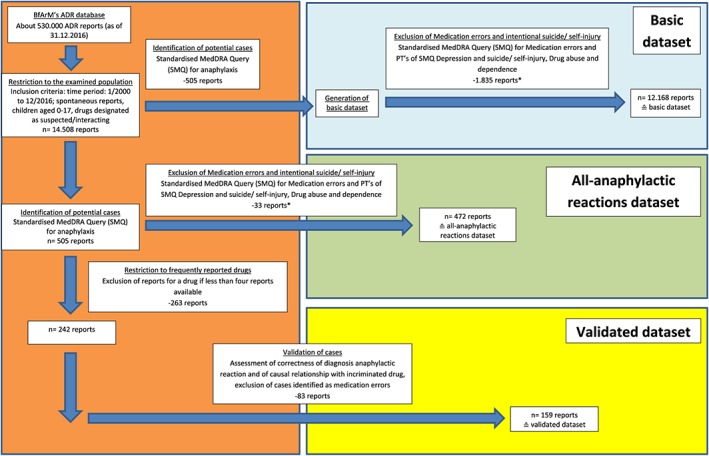
Flow chart depicting the process of identification, selection, and validation of spontaneous reports of anaphylactic reactions contained in the Federal Institute for Drugs and Medical Devices (BfArM's) adverse drug reaction (ADR) database and description of the three datasets (*since cases in which the ADR resulted from a medication error had been deleted from the validated cases, such reports [medication errors or intentional overdose, eg, suicide] were also excluded in the other two datasets by applying respective SMQs. The reasoning for this approach was that, usually, in these cases, inappropriate doses are administered, resulting in a higher risk for ADRs) [Colour figure can be viewed at wileyonlinelibrary.com]
