Figure 3.
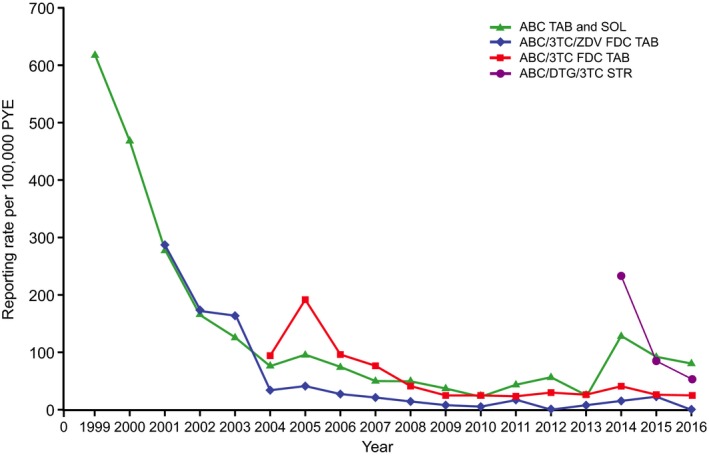
Annual spontaneous reporting rates for all cases fulfilling the Company ABC HSR case definition to December 31, 2016, for each individual ABC‐containing product. ABC = abacavir; DTG = dolutegravir; FDC = fixed‐dose combination; HSR = hypersensitivity reaction; PYE = patient‐years of exposure; SOL = solution; STR = single‐tablet regimen; TAB = tablet; 3TC = lamivudine; ZDV = zidovudine. Cumulative PYE to December 31, 2016, for ABC = 837,429, ABC/3TC/ZDV = 510,913, ABC/3TC = 1,419,426, and ABC/DTG/3TC = 204,844.
