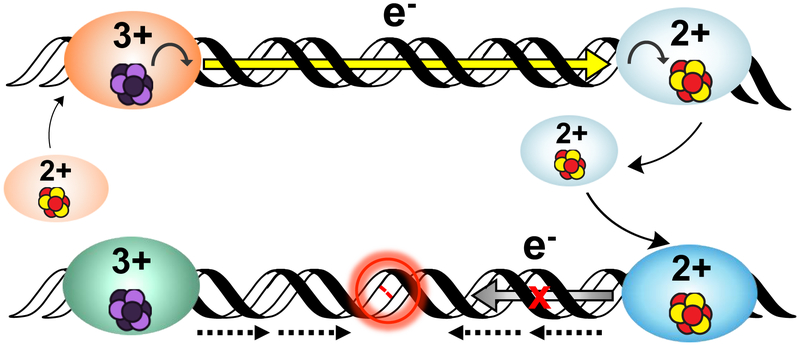
Figure 6. A model for DNA-mediated redox signaling between repair proteins.
Enzymes with the cluster in the native [4Fe4S]2+ state first bind DNA, causing the cluster to become activated toward oxidation. Oxidative stress initiates the damage search when highly reactive species such as the guanine radical cation are formed; these can oxidize DNA-bound proteins in their vicinity. Oxidation of the cluster to the [4Fe4S]3+ state leads to a > 500-fold increase in DNA binding affinity, so oxidized proteins remain bound and diffuse along the DNA. Another [4Fe4S]2+ protein bound at a distant site can reduce the oxidized protein, effectively scanning the intervening DNA for lesions through DNA-mediated CT. At this point, on damage-free DNA (above) the reduced protein binds less tightly to DNA and can diffuse away, while the newly oxidized protein continues the damage search. This process of redox exchange continues until a segment of DNA containing a lesion is approached. Since even subtle lesions can disrupt base stacking (below), CT is attenuated and any nearby oxidized proteins remain bound. Thus, DNA CT allows repair proteins to scan large sections of the genome and redistribute to areas containing damage (24, 37).