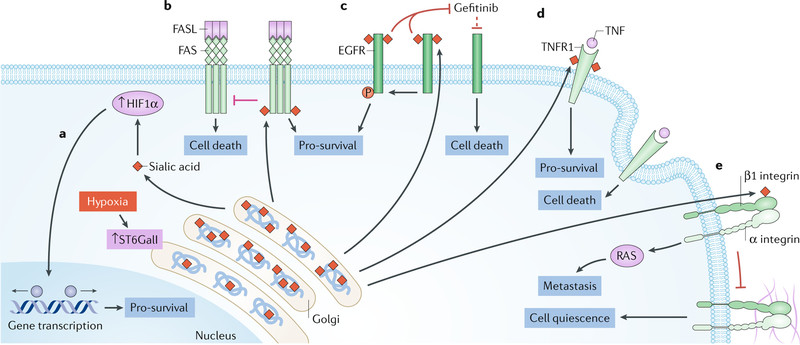
Fig. 5 |. ST6galI and abnormal sialylation in cancer.
N-glycans with terminal α2,6-sialylation are synthesized by the β-galactoside α−2,6-sialyltransferase 1 (ST6GalI). Both upregulation of ST6GalI and increase in α2,6-sialylation are observed in many types of cancers and are associated with negative patient outcomes. In fact, α2,6-sialylation is involved in the regulation of many key proteins that are known to contribute to cell survival and metastasis in cancer. a | Hypoxia increases the expression and activity of ST6GalI, leading to enhanced α2,6-sialylation; in turn, increased sialylation upregulates HIF1α and the expression of pro-survival HIF1α target genes, such as growth factors and glucose transporters. b | The death receptor FAS, also known as CD95, is a target of ST6GalI, and its sialylation inhibits the initiation of apoptotic signalling and subsequent receptor internalization. Increased expression of ST6GalI prevents FAS ligand (FASL)-induced apoptosis through FAS. c | Increased expression of ST6GalI in cancer cell lines enhances the α2,6-sialylation of epidermal growth factor receptor (EGFR), which increases its tyrosine kinase activity and the phosphorylation of its targets279. α2,6-Sialylation enhances the activity of EGFR, both at baseline and after cell activation, and leads to increased activation of pro-growth and survival genes. Moreover, cells in which ST6GalI is overexpressed, leading to enhanced α2,6-sialylation, are protected against cell death induced by the anticancer drug gefitinib, an EGFR inhibitor. d | In cells with low ST6GalI expression and reduced α2,6 sialylation, prolonged activation of tumour necrosis factor (TNF) receptor 1 (TNFR1) by TNF leads to receptor internalization, caspase activation and cell death. This apoptotic cell death pathway is prevented by enhanced α2,6-sialylation of TNFR. e | α2,6-Sialylation of β1 integrin in the Golgi apparatus by ST6GalI results in hypersialylation, which inhibits β1 integrin binding to matrix proteins such as type I collagen and fibronectin and prevents downstream signalling. These signals maintain cell quiescence, and their disruption due to enhanced sialylation leads to increased cell motility and invasion, which promotes cancer cell metastasis.