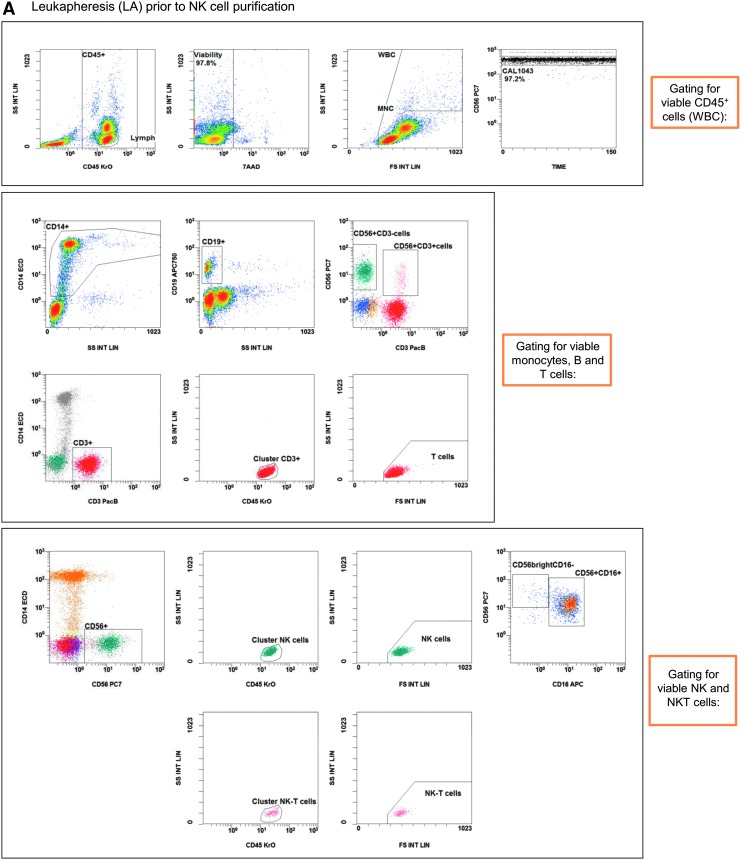
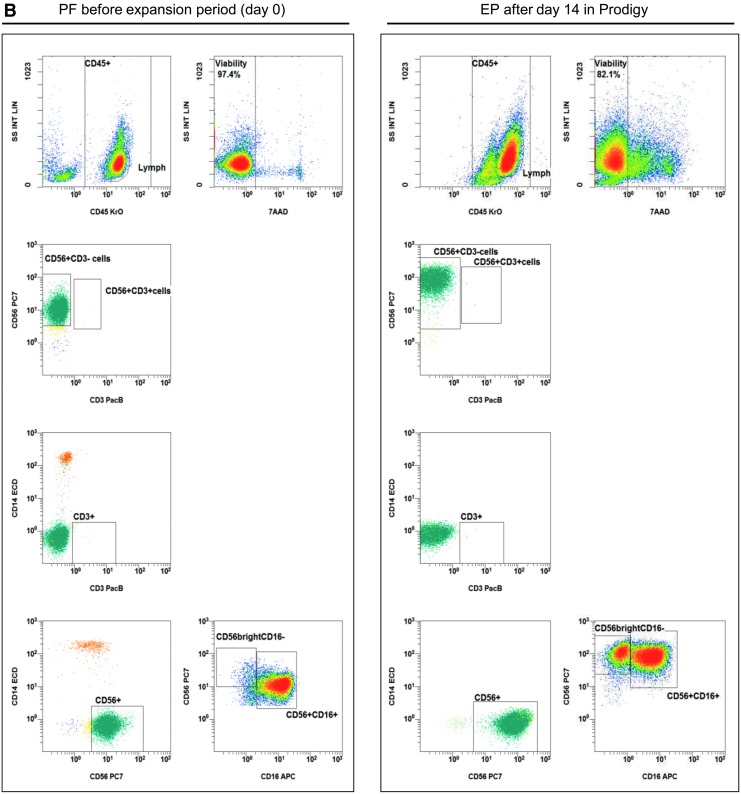
Figure 2.
Gating strategy: flow cytometric quality controls for the quantification of CD3–CD56+ NK cells. IPCs from different process fractions (LA, PF, and EP) were stained by monoclonal antibodies (mABs) to visualize CD3–CD56+ NK cells and analyzed by a no-wash, single-platform method based on an eight-color flow cytometric panel. (A) Initial, CD45−, 7-AAD+, and non-specifically stained debris discriminated by low forward and side scatter (FS/SS) signals were excluded from viable CD45+ cells (WBC). The CAL histogram describes event number of the region “beads” along the time frame to calculate the events for cells/μL and to detect consistent sample flow (upper row, right plot). To identify monocytes and T and B cells, pre-analyzed viable CD45+ cells were used to detect CD14+ monocytes and lymphocyte subpopulations based on CD3 or CD19 surface levels. FS/SS dot plots were gated on the region “MNC” to discriminate nonspecific cell debris and outline the corresponding lymphocyte regions. Gating for viable NK and NKT cells were performed by discrimination (second line: CD3 vs. CD56) of CD3+CD56+ NKT cells to identify CD3−CD56+ NK cells (LA, PF, and EP). (B) One NK manufacturing and expansion process is exemplarily presented. Shown are analyses of PF and EP and the gating strategy for the initial immunomagnetic separation (day 0) and expansion of activated NK cells (day 14).