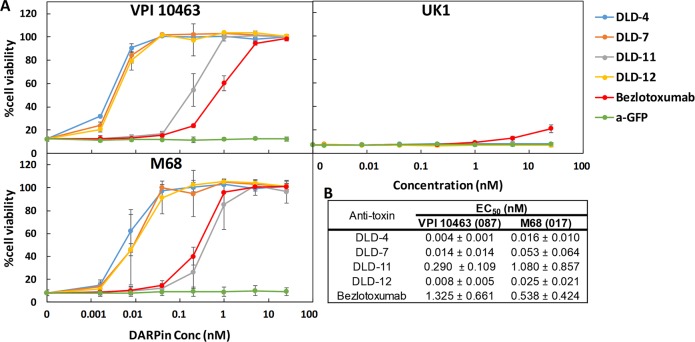
Fig 5. DARPin dimers offer superior protection to Vero cells against the toxicity of TcdB from C. difficile strains VPI 10463 (ribotype 087) and M68 (ribotype 017).
(A) IMAC-purified DARPins were added to Vero cells (1.5 × 103 cells/well) together with TcdB toxin (2.5 pg/mL). Cell viability was quantified 72 hours later by the CellTiterGlo assay and normalized to naïve Vero cells. Error bars represent the standard deviation of at least 2 independent experiments done in duplicate. Bezlotoxumab is the FDA-approved monoclonal antibody for treating recurrent CDI [7]. a-GFP is a GFP-binding DARPin [15] and was used here as a negative control. (B) DARPin dimer TcdB-neutralization potency. Data are the averages of at least 2 independent experiments. CDI, Clostridium difficile infection; Conc, concentration; DARPin, designed ankyrin repeat protein; EC50, half maximal effective concentration; FDA, Food and Drug Administration; IMAC, Immobilized metal affinity chromatography; TcdB, C. difficile toxin B.