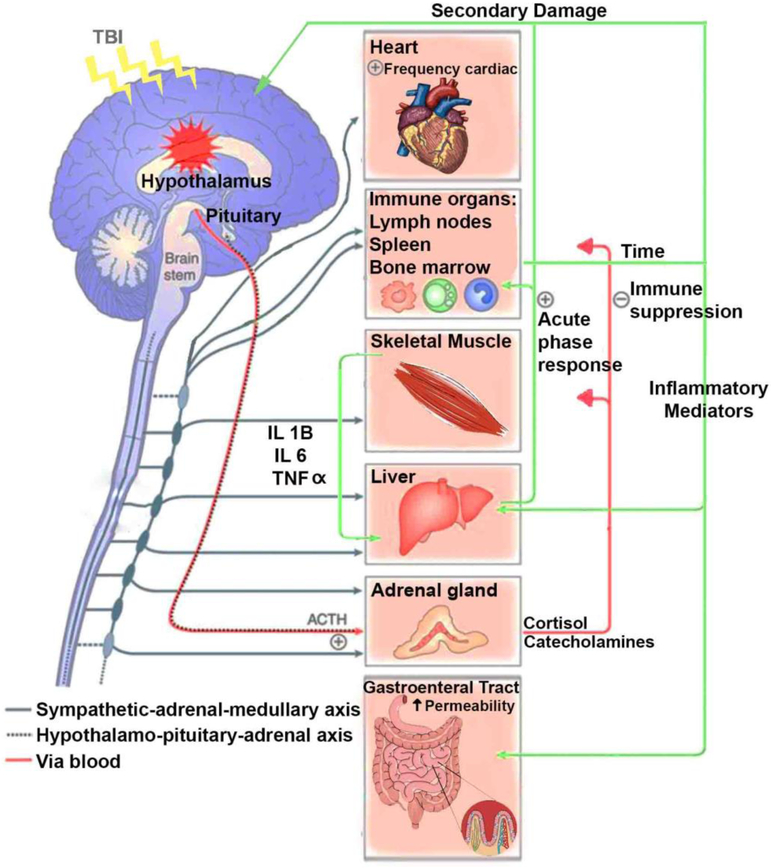
Figure 1.
The diagram illustrates the main organ components for the interaction between the brain and periphery after TBI. TBI induces central inflammation and triggers a multiorgan inflammatory response, involving the action of the autonomic nervous system and hypothalamic-pituitary axis. Autonomic dysregulation and subsequent secretion of catecholamines into the periphery are major players on the generalized host stress response after TBI. For instance: Sympathetic hyperactivity after TBI results in an elevated heart rate and blood pressure. Catecholamine signal through α- and β-adrenergic receptors which are expressed in immune organs like lymph nodes, spleen and bone marrow have the potential to influence the production of inflammatory mediators that alter the redox status in the liver and increase the intestinal permeability after TBI. The generalized sepsis response caused by translocation of bacteria induces a systemic inflammatory sequel with subsequent secondary inflammation in the brain. The liver reacts to sympathetic activation after TBI by displaying a systemic acute-phase response, involving leukocyte mobilization, and increase in cytokines that penetrate the leaky BBB, and become a major player of the secondary brain damage. Indeed, the muscle wasting during the initial phase of injury has been associated with ACTH and catecholamine release since muscle release of amino acids are used for inflammatory protein synthesis in the liver. The Catecholamine and cortisol effects on immune organs like lymph nodes, spleen and bone marrow also contribute to secondary damage after TBI.