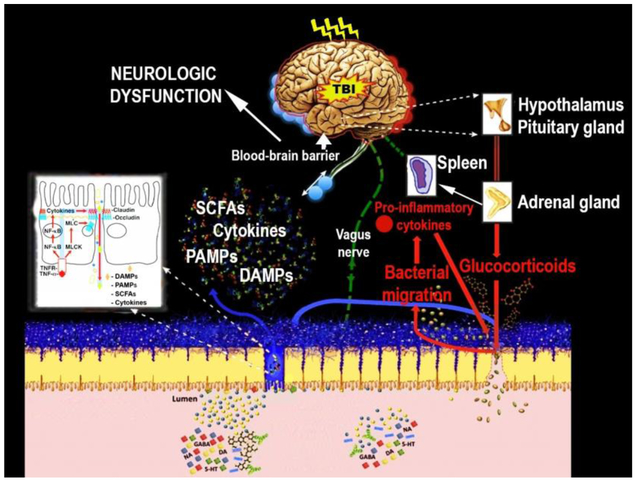
Figure 3.
Proposed mechanism by which TBI alters the brain-gut axis, and in turn, how peripheral pathological signals impact the brain. Right after TBI, dysfunctional hypothalamic-pituitary-adrenal axis disrupts metabolic balance and increases stress hormones such adrenocorticotrophic and cortisol. It is postulated that the ACTH-induced Epinephrine (E) and norepinephrine (NE) by adrenal gland together with efferent vagal output promote splanchnic hypoperfusion, thereby increasing Cytokines and chemokines. The secreted TNF-α by spleen macrophages after TBI binds TNF receptors (TNFRs) on intestinal epithelial cells and activates several pathways, including the NF-κB pathway that upregulates genes encoding pro-inflammatory cytokines and myosin light chain kinase (MLCK). These signals enhance tight junction permeability in the intestine involving damage-associated molecular patterns (DAMPs), Pathogen-associated molecular patterns (PAMPs), and cytokines. The Cytokines produced increases intestinal permeability and then penetrate the leaky BBB to contribute to secondary brain damage characterized by fatigue, and learning and memory dysfunctions.