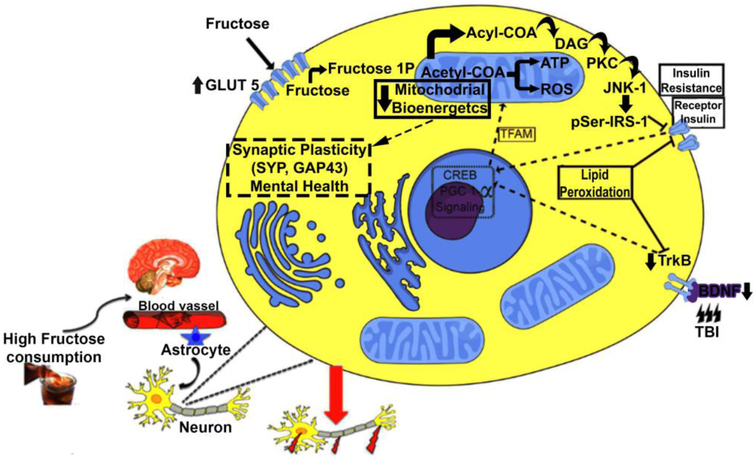
Figure 5.
Proposed mechanism by which metabolic disorders like the one elicited by high fructose consumption may aggravate the pathophysiology of TBI. In CNS, fructose increases levels of the fructose transporter GLUT5 suggesting that fructose consumption enhances its own transport into the brain. We propose that intracellular fructose accumulation induces the formation of acetyl-CoA and acyl-CoA. High levels of acyl-CoA can be converted to diacylglycerol (DAG) that activates the protein kinase C epsilon (PKCε), which, in turn, activates the protein c-jun-N terminal kinase-1 (JNK1). This protein leads to insulin resistance through the phosphorylation of IRS-1 on Serine307 residue (IRS-1Ser307). It is well know that the TrkB BDNF receptor and InR receptors are involved in regulation of cell metabolism and synaptic plasticity in neurons. Along this line of thought, the actions of fructose and TBI converge and inhibit pathways associated with management of cell energy metabolism like cAMP response element-binding protein (CREB) and peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α). Considering the interactive actions of PGC-1α and mitochondrial transcription factor A (TFAM) on mitochondrial biogenesis, it is plausible to propose that fructose and TBI decrease oxidative phosphorylation and bioenergetics. It is import to note fructosylation releases large amounts of superoxide anions, leading to disproportionate formation of reactive oxygen species in mitochondria. Moreover, the loss in energy homeostasis results in ROS, and the harmful by-product of lipid peroxidation 4-hydroxynonenal (4HNE), thereby compromising plasma membrane function. Therefore, a high fructose diet and TBI disrupt the interplay between energy metabolism and synaptic plasticity with profound consequences for brain function.