Figure 1.
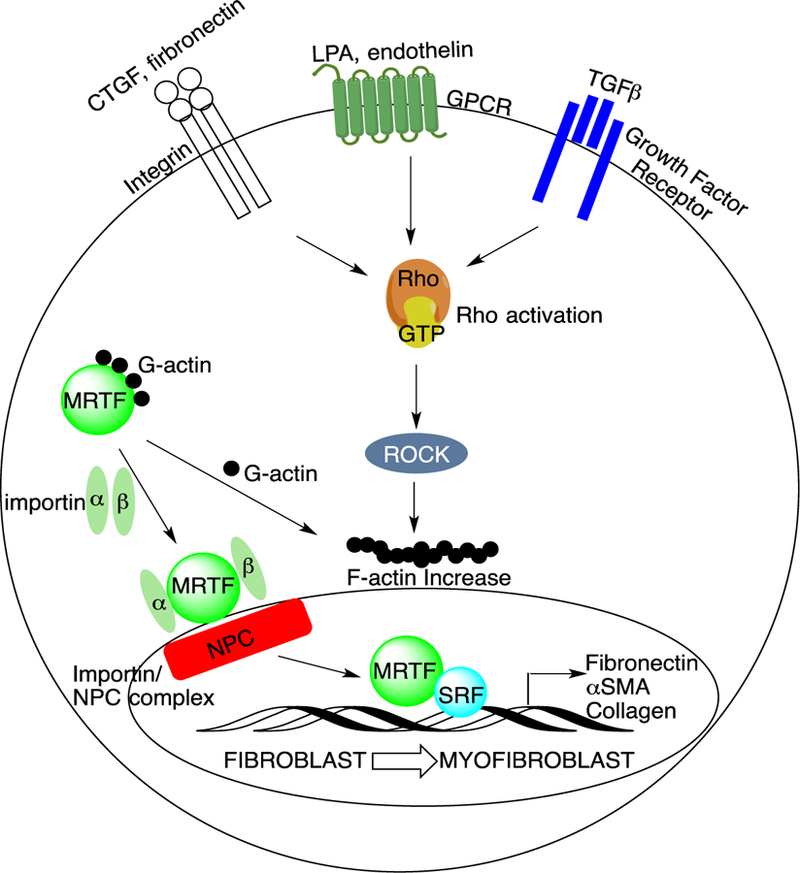
The fibroblast-to-myofibroblast transition is tightly regulated by Rho/MRTF/SRF signaling. Both soluble and mechanical stimuli promote MRTF nuclear translocation in fibroblasts. In the resting state, actin is not polymerized, and MRTF is bound to G-actin and sequestered into the cytoplasm. When stress fibers form in response to these stimuli, G-actin polymerizes into F-actin and MRTF is released. This allows MRTF to translocate into the nucleus via binding to importins followed by MRTF/importin/nuclear pore complex (NPC) formation. The MRTF/SRF coactivator complex increases ECM and pro-fibrotic gene expression, contributing to the fibroblast-to-myofibroblast transition.
