Scheme 1. General Synthesis of 5-Aryl-1,3,4-oxadiazol-2-ylthioalkanoic Acidsa.
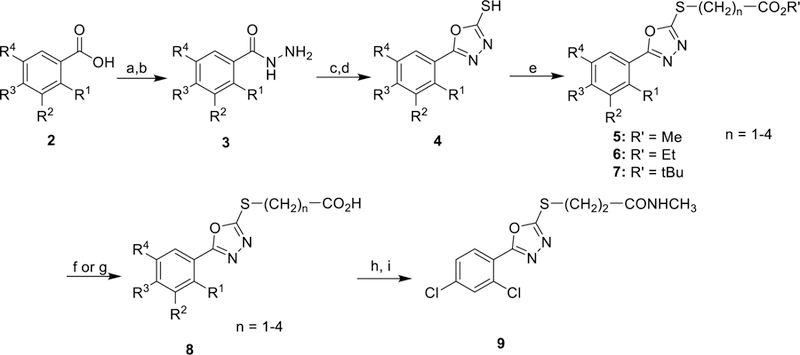
aReagents and conditions: (a) H2SO4/MeOH, 85 °C 6 h. (b) H4N2•H2O, 85 °C 14 h. (c) KOH/H2O/EtOH, CS2, 95 °C 16 h. (d) 1N HCl workup (e) acetone, K2CO3, Br-(CH2)n-CO2R’, 25 °C, 5–24 h. (f) DCM, TFA, 25 °C 3 h. (g) 1M NaOH, THF, 25 °C 16 h. (h) oxalyl chloride, DMF, CH2Cl2, 25 °C, 2 h. (i) MeNH2 in EtOH, 25 °C, 16 h.
