Scheme 3. Synthetic Route to Generate Thiobutanoate Analogs 19a-ia.
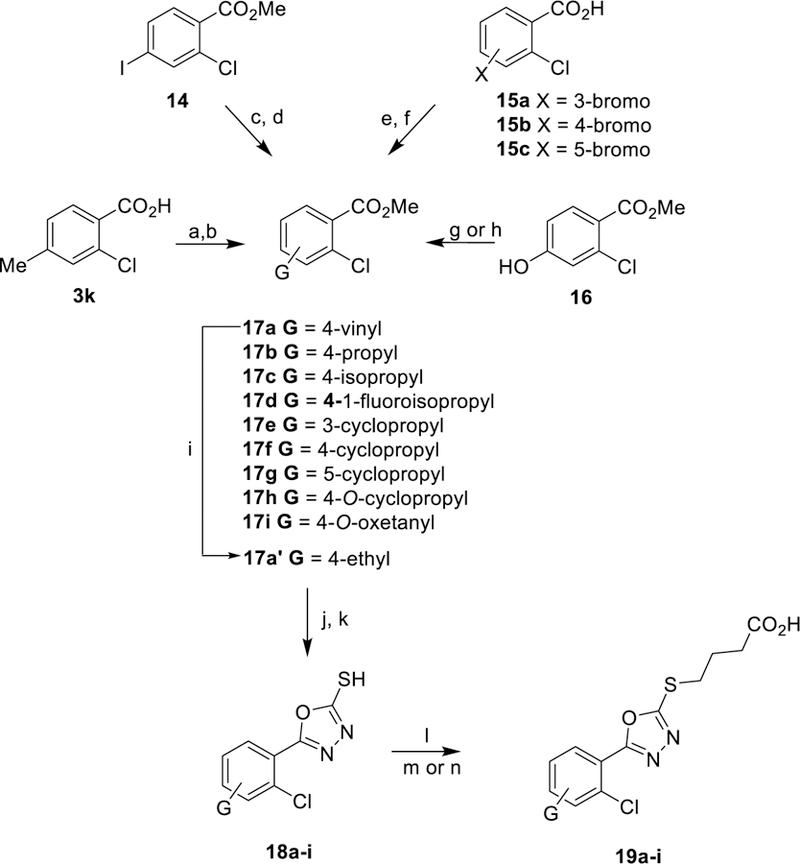
aReagents and conditions: (a) LDA (2 eq.), THF, 0 °C, 10 min, then ethyl iodide, 0 °C, 1 h. (b) MeOH, H2SO4, 85 °C, 16 h. (c) iPrMgCl, THF, −20 °C for 1.5 h, then acetone, 25 °C for 1.5 h. (d) DAST, DCM, −78 °C to 25 °C 2 h. (e) MeOH, H2SO4, 85 °C, 16 h. (f) Pd(II)OAc2Cl2, K3PO4, P(Cy)3, cyclopropyl boronic acid, toluene, H2O, 100 °C, 3 h. (g) bromocyclopropane, Cs2CO3, DMA, 155 °C, 24 h then MeOH, H2SO4, 85 °C, 16 h. (h) 3-bromooxetane, Cs2CO3, DMSO, 105 °C, 8 h. (i) 2-nitrobenzene-1-sulfonyl chloride, H2N4•H2O, MeCN, 0 °C to 25 °C, 16 h. (j) H4N2•H2O, MeOH, 85 °C, 16 h. (k) KOH/H2O/EtOH, CS2, 95 °C, 16 h., followed by HCl workup (l) 4-bromo-butanoate ester, K2CO3, acetone, 25 °C, 16 h. (m) 1M NaOH, THF, 25 °C, 1 h. (n) TFA, DCM, 25 °C, 1 h.
