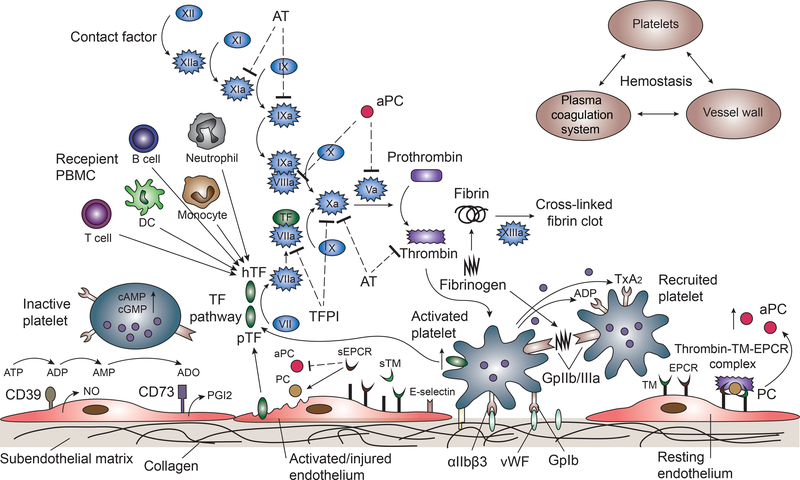
Figure 3: The coagulation cascade relating to pig liver xenotransplantation is illustrated.
The extrinsic pathway is initiated by tissue factor (TF), which forms complexes with factor VIIa, activating factor X, which in turn activates thrombin downstream. Thrombin converts factor XI to XIa, VIII to VIIIa, V to Va, fibrinogen to fibrin, and XIII to XIIIa, and promotes platelet activation and aggregation. Glycoprotein 1b (GpIb) is a component of the GPIb-V-IX complex on platelets, which binds von Willebrand factor (vWF), allowing platelet adhesion. ADP activates platelets to change conformation of GpIIb/IIIa receptors, which bind to fibrinogen to mediate platelet aggregation and endothelial adherence. Protein C is activated by the thrombin-thrombomodulin (TM)-endothelial protein C receptor (EPCR) complex. (TM serves as a cofactor in the thrombin-induced activation of protein C. EPCR is a receptor for protein C that enhances its activation). Activated protein C and its cofactor, protein S, deactivate factor Va and VIIIa. Tissue factor pathway inhibitor (TFPI) rapidly inhibits the TF/VIIa complex, and deactivates factor Xa. Anti-thrombin (AT) inhibits factors IXa, Xa, XIa, and thrombin via a complex formation with its active site. CD39 hydrolyzes ATP and ADP to AMP, and CD73 converts AMP to adenosine (ADO), to inactivate platelets.
(Other abbreviations: hTF=human TF; pTF=pig TF; sTM=soluble TM; sEPCR=soluble EPCR; PGI2= prostaglandin I2; TxA2=thromboxane A2; PBMC=peripheral blood monocular cells.)